Schwertmannite prepared by alkali neutralization method and application of schwertmannite
A technology of schwittmannite and application method, applied in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc., can solve the problems affecting the preparation rate of schwittmannite, small specific surface area, unfavorable Cr adsorption And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides the preparation of Schwittmannite by alkali neutralization method, and the specific steps are as follows:
[0042] Configure 80mmol / L of Fe in the Erlenmeyer flask 2+ (as FeSO 4 is the iron source) solution, to the Fe-containing 2+ Add 1.60mL H to the solution 2 o 2 , at H 2 o 2 After adding, adjust the pH to 2.7 with lye (5MNaOH) every 30min, and react in a shaking table at 180r / min for 24 hours; after the product is collected by membrane filtration and washed several times, an improved Schwittmannite adsorbent is obtained. Samples were preserved after vacuum freeze-drying.
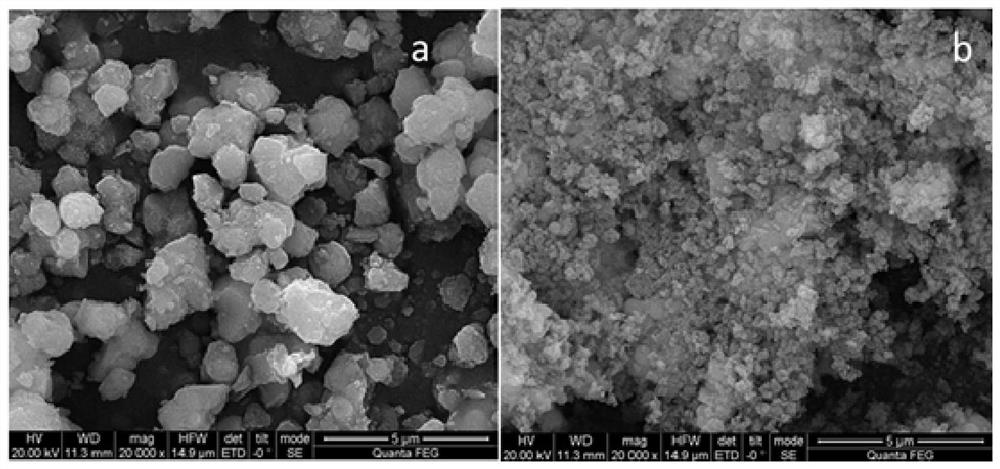
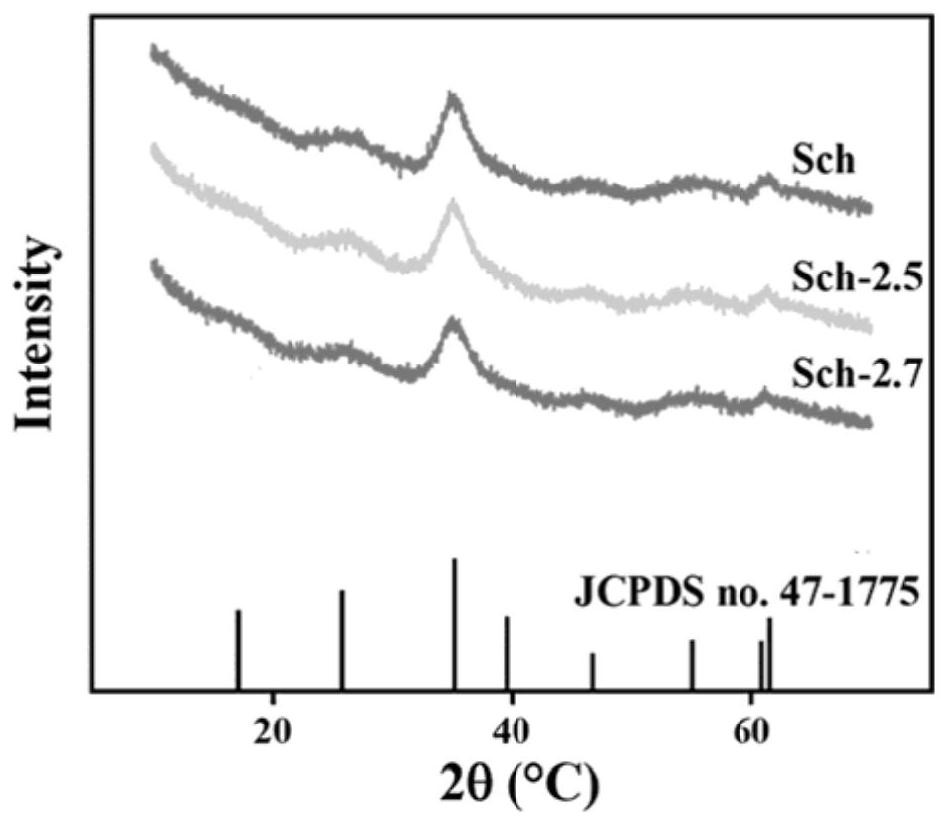
[0043] Schwittmann's stone synthesized by alkali neutralization figure 1 (b), from figure 1 From the SEM spectrum of b, it can be seen that the synthesized Schwittmannite is a solid particle with a smooth spherical appearance and a size of 100nm; figure 2 The XRD pattern verified that the synthesized Schwittmannite (the characteristic peaks of 26.26°, 35.16°, 4...
Embodiment 2
[0045] This embodiment provides the preparation of Schwittmannite by alkali neutralization method, and the specific steps are as follows:
[0046] Configure 80mmol of Fe in the Erlenmeyer flask 2+ (as FeSO 4 is the iron source) solution, to the Fe-containing 2+ Add 1.45mLH to the solution 2 o 2 , at H 2 o 2 After the addition, the pH was adjusted to 2.5 with lye (5M NaOH) every 40 minutes, and reacted on a 160r / min shaker for 20 hours; the product was collected by membrane filtration and washed several times to obtain an improved Schwittmannite adsorbent. Samples were preserved after vacuum freeze-drying.
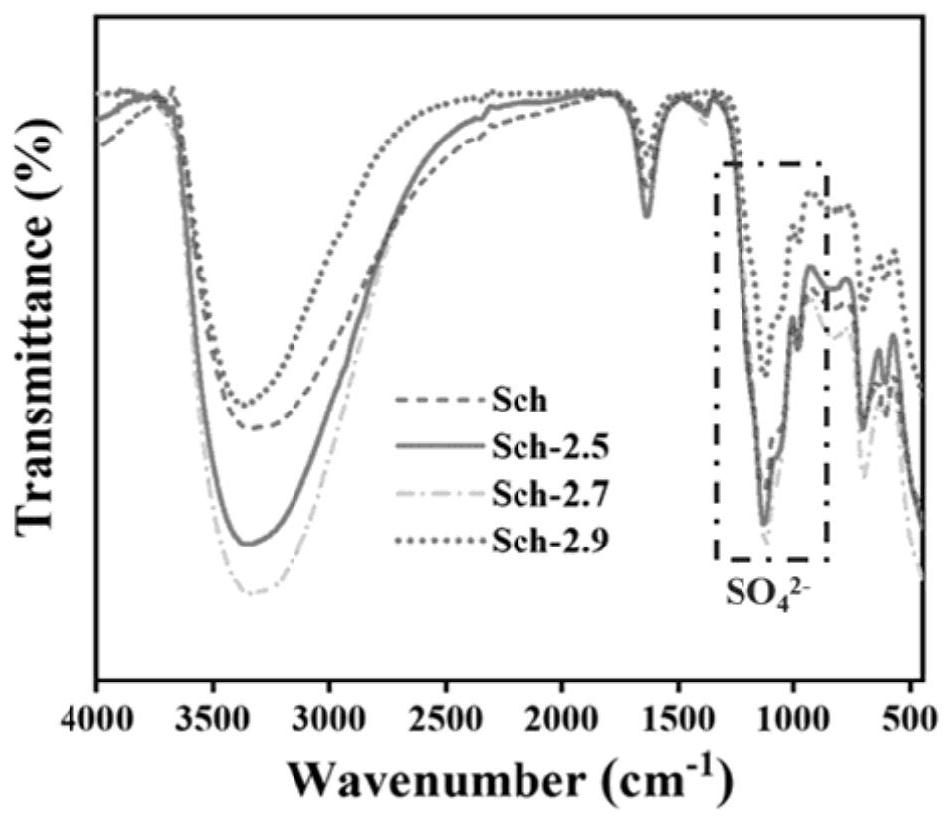
[0047] figure 2 The XRD figure of verification is that Schwittmannite (26.26°, 35.16°, 46.53° and 61.34° characteristic peaks correspond to the standard card of Schwittmannite respectively) shows that Schwittmannite is successfully prepared. The particles prepared by Schwittmannite are smaller, with a size of 150nm; image 3 The infrared analysis spectrum in shows...
Embodiment 3
[0058] The present embodiment provides the application of the Schwittmannite prepared by the alkali neutralization method in removing Cr(VI) in water, which specifically includes the following steps:
[0059] Prepare Cr(VI) (concentration is 100mg / L) solution in the reaction bottle, adjust the pH to be 3, then add 1g / L Schwittmannite prepared in Example 1; carry out adsorption experiment at room temperature 25 ℃, reach adsorption equilibrium Then the Cr(VI) adsorption amount was determined.
[0060] from Figure 5 It can be seen that when pH=3, the dissolved SO of the Schwittmannite prepared by alkali neutralization 4 2- 26.78mg / g; Dissolution of SO from ordinary Sch prepared without pH control 4 2- The amount is 12.41mg / g, indicating that the Schwittmannite prepared by alkali neutralization and pH control dissolves more SO 4 2- , which is conducive to the occurrence of ion exchange, which is conducive to the adsorption of more Cr(VI), such as Figure 4 As shown, the ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com