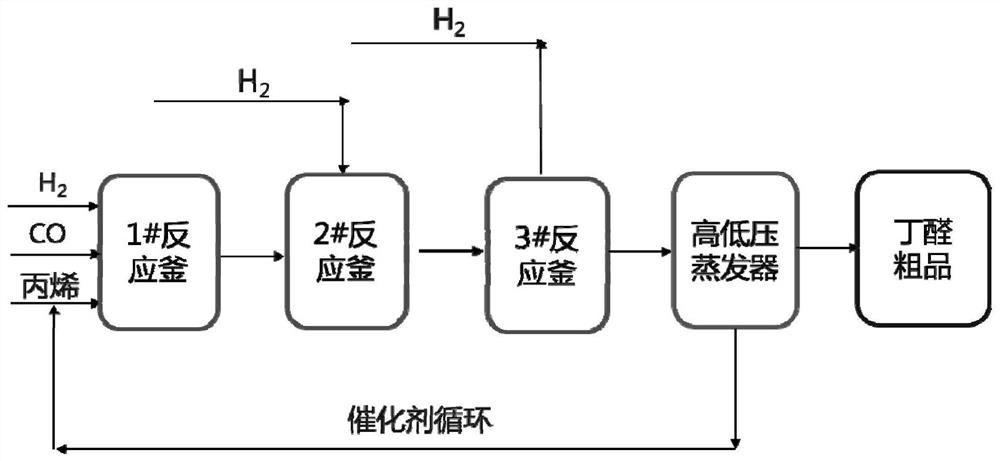
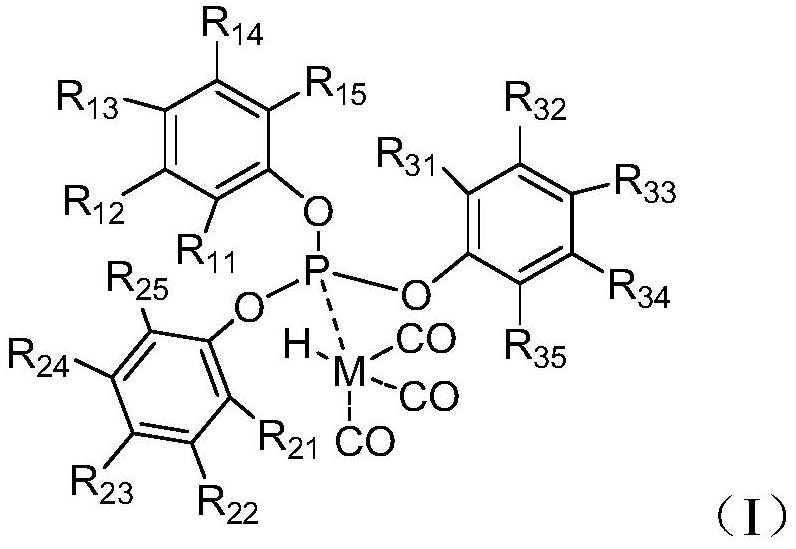
Olefin hydroformylation catalyst composition and hydroformylation process
A technology of hydroformylation catalysts and compositions, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem that 2-methylbutyraldehyde cannot achieve Intermittent response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation method of the monodentate complex of the present invention is not particularly limited, and may be a conventional preparation method known in the art. In an example of the present invention, the preparation method includes dissolving a ligand (eg, ligand L-3) and a metal compound (eg, rhodium dicarbonyl acetylacetonate) with a solvent (eg, aromatic hydrocarbon, valeraldehyde, etc.) in a certain proportion , added to the autoclave. After the synthesis gas is introduced for replacement (for example, 3 times of replacement), the pressure is charged (for example, the pressure is charged to 1-2 MPaG, preferably 1.5 MPaG), and the reaction is heated (for example, heated to 60-90 ° C, preferably 80 ° C for 0.5-2 hours. , preferably 1 hour), that is, the formation of a monodentate complex (eg, a monodentate complex I-3).
[0054] In one example of the present invention, suitable solvents are neutral solvents such as benzene, toluene, xylene or mixtures thereof,...
Embodiment 1
[0116] Into a 200mL stainless steel autoclave equipped with a pressure gauge, add 100g of anhydrous toluene solution dissolved with the following catalyst components:
[0117]
[0118]The molar ratio of Rh / P was controlled to be 1:18, the concentration of Rh was 60 ppm, and the molar ratio of rhodium complex to free phosphite ligand was 1:7. After replacing the gas in the kettle three times with synthesis gas (hydrogen: carbon monoxide=1:1), add 10 g of propylene, stir with an electromagnetically driven mechanical stirrer, feed the synthesis gas to a pressure of 0.5 MPaG in the kettle, and heat up to a temperature of 80 °C in the kettle. ℃, feed synthesis gas (hydrogen: carbon monoxide = 1:1) to a total pressure of 1.5 MPaG, maintain the temperature and pressure in the kettle for 0.3 h, and calculate on the basis of propylene, the conversion rate is 98%, and the total amount of butyraldehyde generated in the reaction is 98%. The selectivity was 98.9%, and the molar ratio of...
Embodiment 2
[0120] Into a 200mL stainless steel autoclave equipped with a pressure gauge, add 100g of anhydrous toluene solution dissolved with the following catalyst components:
[0121]
[0122] The molar ratio of Rh / P was controlled to be 1:18, the concentration of Rh was 60 ppm, and the molar ratio of rhodium complex to free phosphite ligand was 1:7. After replacing the gas in the kettle three times with synthesis gas (hydrogen: carbon monoxide=1:1), add 10 g of propylene, stir with an electromagnetically driven mechanical stirrer, feed the synthesis gas to a pressure of 0.5 MPaG in the kettle, and heat up to a temperature of 80 °C in the kettle. ℃, feed synthesis gas (hydrogen: carbon monoxide = 1:1) to a total pressure of 2.0 MPaG, maintain the temperature and pressure in the kettle for 0.3 h, and calculate with propylene as the benchmark, the conversion rate is 97.4%, and the total amount of butyraldehyde generated by the reaction is 97.4%. The selectivity was 98.1%, and the mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com