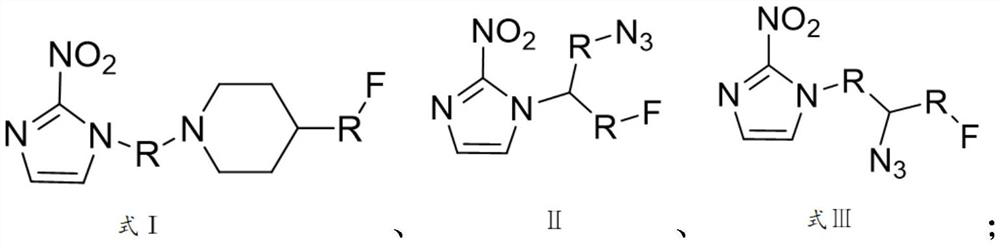
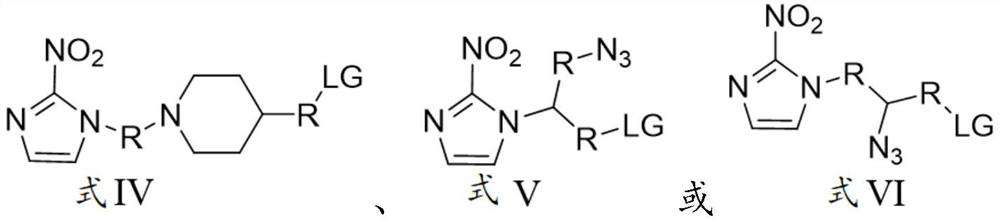
Nitroimidazole derivative for preparing hypoxic developer, preparation method and application thereof
A technology of nitroimidazoles and derivatives, which is applied in the fields of medical imaging materials and medicinal chemistry, can solve the problems of low brain intake, low hypoxia sensitivity, and low brain intake.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1 1-(4-(2-fluoroethyl)piperidin-1-yl)-2-(2-nitro-1H-imidazol-1-yl)ethan-1-one
[0036] The structural formula is as follows:
[0037]
[0038] The synthetic route is as follows:
[0039]
[0040] 1) 2-(2-nitro-1H-imidazol-1-yl)-1-(4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperidine- 1-yl)ethane
[0041]Dissolve 2-(2-nitro-1H-imidazol-1-yl)acetic acid (120mg, 0.70mmol) in DMF, add 5mL triethylamine, stir at room temperature for 15min, then add 4-(2-((tetrahydro -2H-pyran-2-yl)oxy)ethyl)piperidine (150mg, 0.70mmol), 1-hydroxybenzotriazole (HOBT, 30mg, 0.19mmol), O-benzotriazole-tetra Methylurea hexafluorophosphate (HBTU, 800mg, 2.11mmol), react at room temperature for 1h, then extract with ethyl acetate, wash with saturated brine three times, collect the organic layer, and wash with Na 2 SO 4 dry. Purification by flash chromatography (ethyl acetate) afforded 2-(2-nitro-1H-imidazol-1-yl)-1-(4-(2-((tetrahydro-2H-pyran-2- yl)oxy)ethyl)piperidin-1-yl)eth...
Embodiment 2
[0048] Example 2 4-(2-fluoroethyl)-1-(2-(2-nitro-1H-imidazol-1-yl)ethyl)piperidine
[0049] The structural formula is as follows:
[0050]
[0051] The synthetic route is as follows:
[0052]
[0053] 1) 2-(1-(2-(2-nitro-1H-imidazol-1-yl)ethyl)piperidin-4-yl)ethan-1-ol
[0054] Dissolve 2-(2-nitro-1H-imidazol-1-yl)ethyl 4-methylbenzenesulfonate (353mg, 1.13mmol) and 4-piperidineethanol (220mg, 1.70mmol) in acetonitrile, add K 2 CO 3 2-(1-(2-( 2-nitro-1H-imidazol-1-yl)ethyl)piperidin-4-yl)ethan-1-ol (200 mg, 66.0%). 1 H NMR (300MHz, CDCl 3 )δ7.14(s,1H),7.00(s,1H),4.42(t,J=6.1Hz,2H),3.55(t,J=6.5Hz,2H),3.04(s,1H),2.68( d,J=11.2Hz,2H),2.58(t,J=6.1Hz,2H),2.01(s,2H),1.56(d,J=12.4Hz,2H),1.39(dd,J=12.7,6.3 Hz,3H),1.14–0.91(m,2H). 13 C NMR (75MHz, CDCl 3 )δ145.00,127.87,126.38,59.88,58.24,54.05,47.41,39.11,32.14,31.95.HRMS calcd for C12H20N4O3 268.1535;found,269.2187[M+H] + .
[0055] 2) 2-(1-(2-(2-(2-nitro-1H-imidazol-1-yl)ethyl)piperidin-4-yl)ethyl 4-methylbenzenesul...
Embodiment 3
[0059] Example 3 1-(4-(2-fluoroethyl)piperidin-1-yl)-3-(2-nitro-1H-imidazol-1-yl)propanol
[0060] The structural formula is as follows:
[0061]
[0062] The synthetic route is as follows:
[0063]
[0064] 1) 2-(1-(3-(2-nitro-1H-imidazol-1-yl)-2-((tetrahydro-2H-pyran-2-yl)oxy)propyl)piperidine- 4-yl)ethanol
[0065] 3-(2-nitro-1H-imidazol-1-yl)-2-((tetrahydro-2H-pyran-2-yl)oxy)propyl 4-toluenesulfonate (150mg, 0.35mmol ) and 4-piperidine ethanol (68mg, 0.52mmol) were dissolved in acetonitrile, added K 2 CO 3 (487mg, 3.52mmol), reflux at 90°C for 24h, then spin dry and mix the sample, and pass through flash purification chromatography (ethyl acetate) to obtain 2-(1-(3-(2-nitro-1H-imidazole-1- yl)-2-((tetrahydro-2H-pyran-2-yl)oxy)propyl)piperidin-4-yl)ethanol (100 mg, 74.8%). 1 H NMR (300MHz, CDCl 3 )δ7.19(s,1H),7.09(s,1H),4.99(dd,J=14.0,2.9Hz,1H),4.36–4.28(m,2H),4.10(d,J=5.0Hz,1H ),3.82–3.65(m,1H),3.82–3.65(m,3H),3.42-3.39(m,1H),2.91(d,J=10.5Hz,1H),2.76(d,J=10.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com