Anti-inflammatory polypeptide DAvp-1 in snake venom and application of anti-inflammatory polypeptide DAvp-1
A technology of davp-1 and anti-inflammatory drugs, applied in the field of molecular biology, can solve the problem of unclear anti-inflammatory components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of a Agkistrodon venom phage library
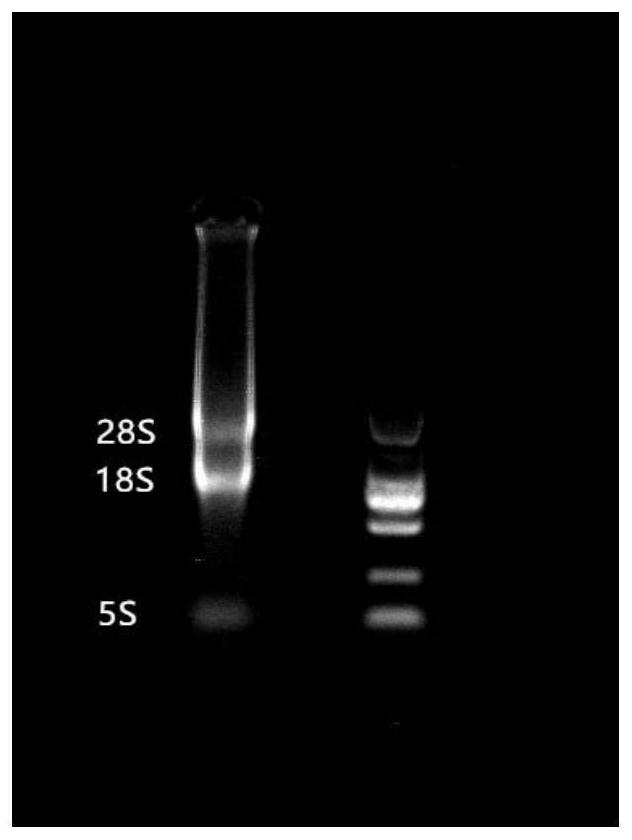
[0029] The overall steps of this embodiment are as follows: firstly extract the total RNA of the venom gland tissue of Agkistrodon acarina, extract mRNA, and obtain cDNA by reverse transcription of the extracted mRNA. A synthetic restriction site is added to the end of the cDNA and digested. DNA fragments that are too low in length are eluted after enzyme digestion. Connect the T7 select vector arms, insert the gene encoding the target peptide into the genome of phage T7, and fuse the cDNA sequence of Agkistrodon venom to the C-terminal of the 10B capsid, so that the target protein is expressed on the surface of the phage particle, and then transduced into E. coli cells.
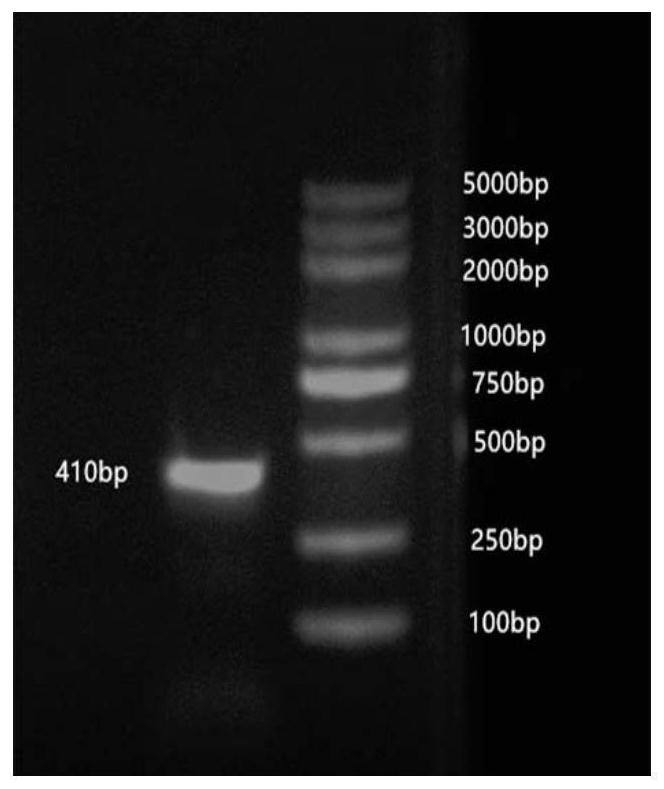
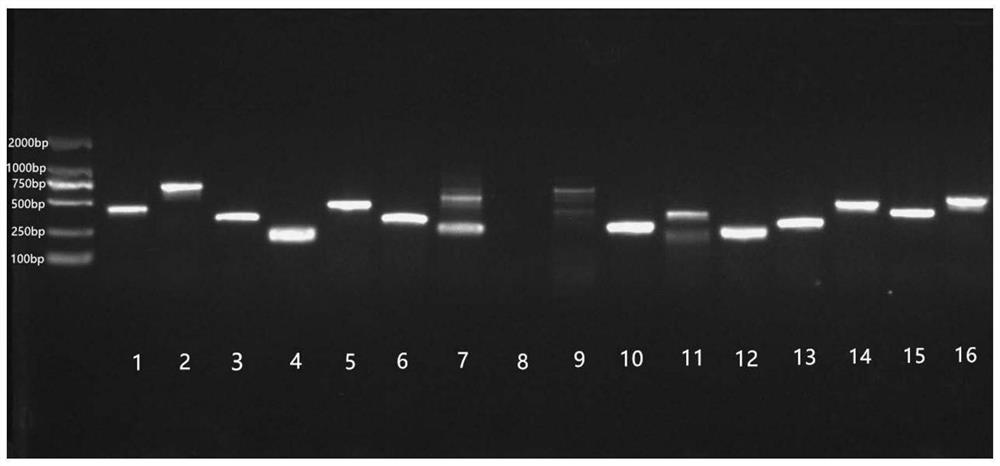
[0030] The initial titer of the obtained phage library was 1.2×10 6 pfu / mL. The phage plaques of the original library were randomly selected, and PCR and gel electrophoresis were performed on the genes contained in the phage plaques. ...
Embodiment 2
[0089] Example 2: Screening of specifically binding phages, analysis and acquisition of anti-inflammatory polypeptide DAvp-1
[0090] In this example, on the basis of the phage display library obtained in Example 1, using human recombinant tumor necrosis factor type I receptor (hrTNFR1) as a substrate, a phage display polypeptide capable of binding to TNFR1 was obtained after three rounds of screening. By sequencing the gradually enriched phage genome, the gene sequence of the phage is obtained, and the amino acid sequence of the peptide displayed on the surface of the phage is translated. Synthesize according to the peptide sequence, and further analyze the binding force between these peptides and TNFR1 and the antagonistic effect on TNF-α, so as to screen and obtain drugs with anti-inflammatory potential.
[0091] The reagents involved in this embodiment are as follows:
[0092] 1. Coating Buffer 1×Coating Buffer: Pipette 5mL of 10×Coating Buffer into a graduated cylinder, ...
Embodiment 3
[0111] Example 3: Protein structure model simulation and binding force detection of anti-inflammatory polypeptide DAvp-1
[0112] 1. Configuration This embodiment involves reagents:
[0113] (1) 1×PBS-EP: Add 450 mL of filtered ultrapure water to 50 mL of 10×PBS-EP.
[0114] (2) Ligand protein TNFR1 solution: protein TNFR1 was diluted with pH=5.5 sodium acetate, pH=5.0 sodium acetate, pH=4.5 sodium acetate to 10 μg / mL and 100 mL respectively. The pH=5.0 was determined as the optimal coupling condition through the pre-enrichment experiment, so the TNFR1 solution was diluted to 10 μg / mL with sodium acetate at pH=5.0, and 200 μL was prepared for formal coupling.
[0115] (3) Prepare peptide solution: add 168 μL water to 0.8 g DAvp-1 to prepare a 1 mM solution.
[0116] 2. Protein structure prediction. Use the protein structure prediction (PSIPRED) server to predict the secondary structure of DAvp-1, the results are as follows Figure 4 As shown, consists of partial helixes an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com