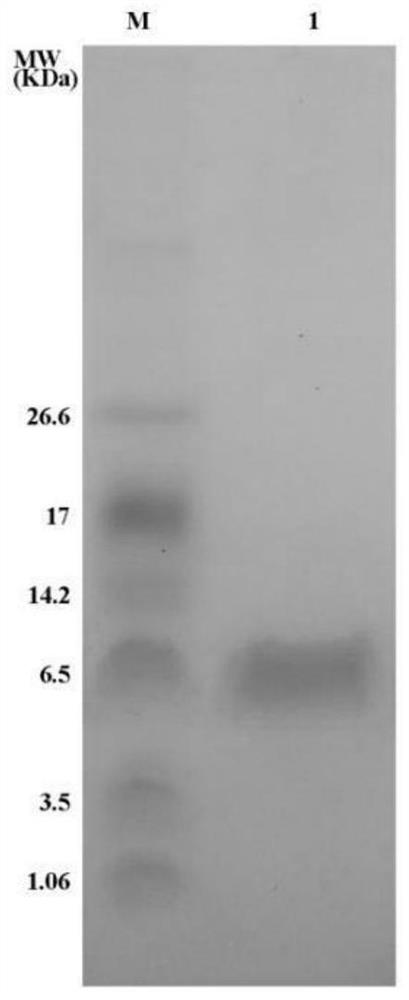
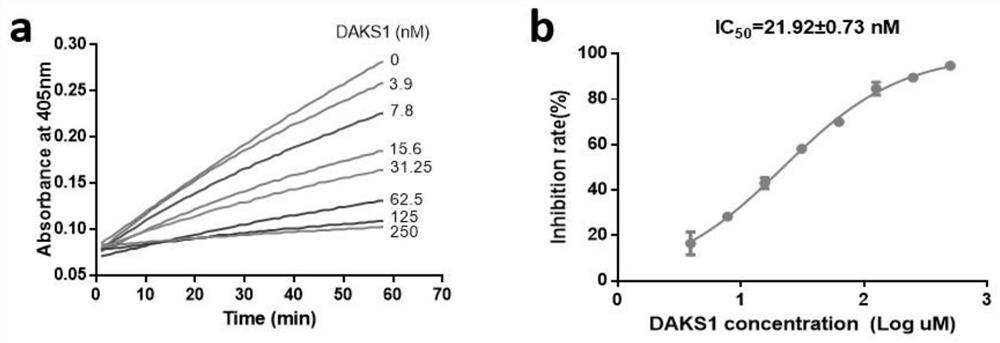
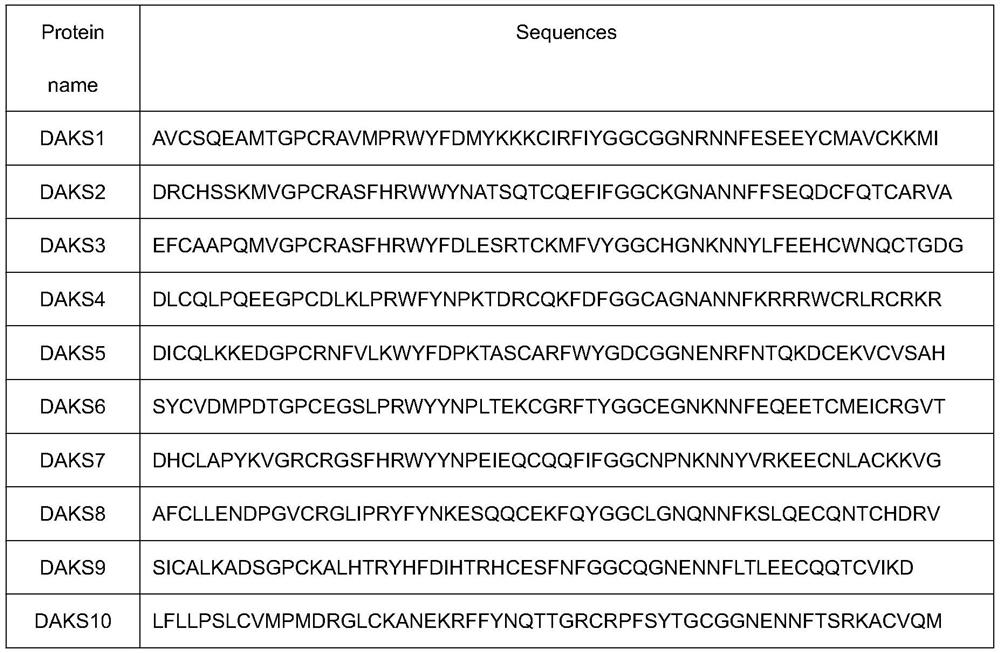
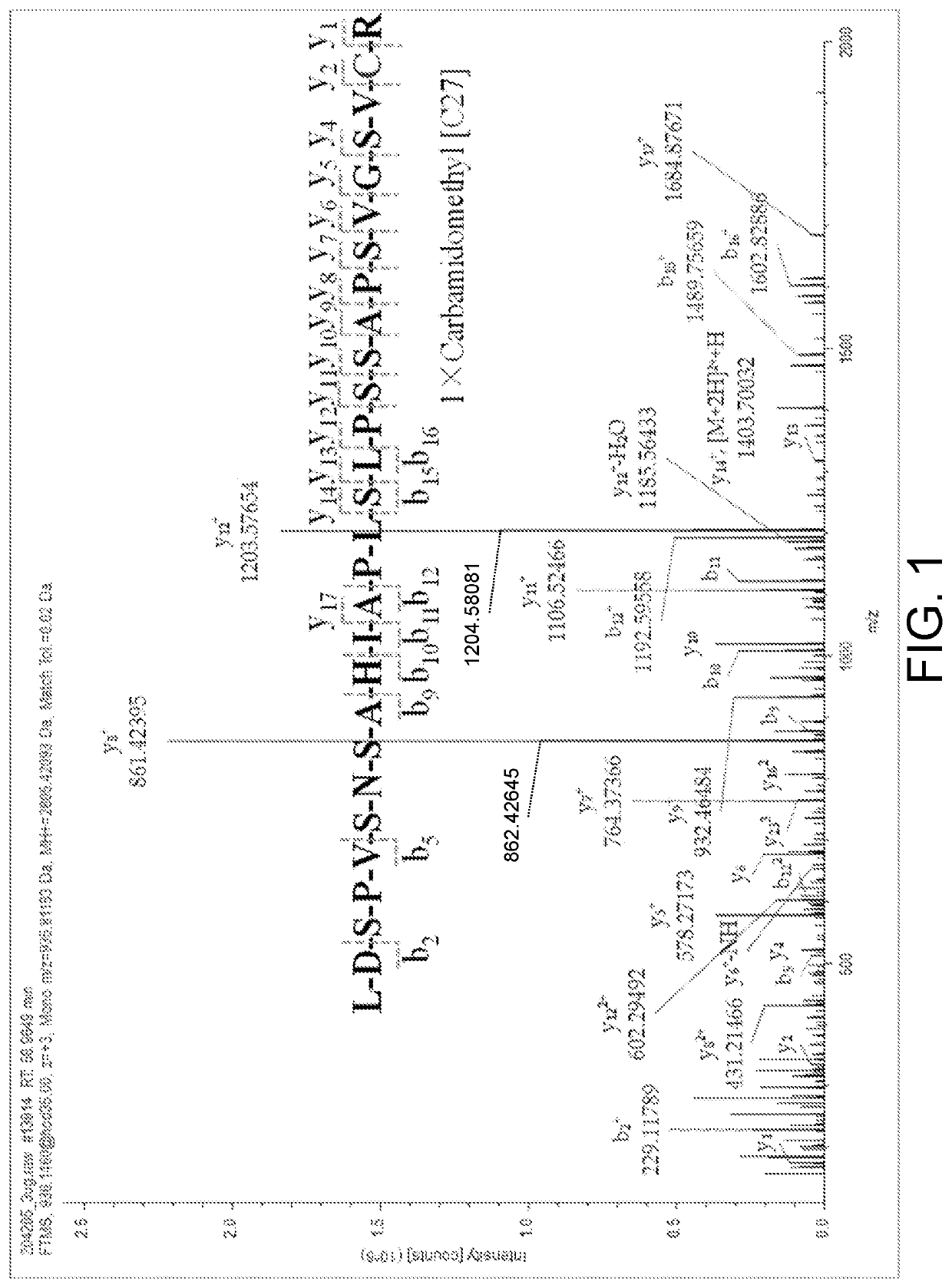
Patents
Literature
32 results about "Agkistrodon blomhoffii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel snake venom polypeptide, preparation and application thereof
InactiveCN101314617AImprove anti-tumor effectEnhanced inhibitory effectPeptide/protein ingredientsPeptide preparation methodsPit viperSnake venom
The invention provides a novel snake venom polypeptide, which is extracted from the coarse venom of pit vipers and has molecular weight of 7554Da. The sequence of 1-15 amino acids at the N terminal is shown in formula (I), wherein E is glutamic acid; A is lactamic acid; D is aspartic acid; N is asparagines; C is cystine; and N represents an amino terminal. The snake venom polypeptide has high anti-tumor activity, and is applicable in preparing anti-tumor pharmaceuticals. The formula (I) is N-EAEEDEDDDAAAANC.
Owner:SOUTHERN MEDICAL UNIVERSITY
Snake venom polypeptide and its preparation method
InactiveCN1800208AGood antitumor activityGrowth inhibitionPeptide preparation methodsAntineoplastic agentsCyanideTrifluoroacetic acid
The invention relates to a venom polypeptide and it's preparing method in the field of biochemistry and biological medicine. The venom polypeptide has the sequence of (I) and the molecular weight is 7480Da. The preparing method is that it dose separating purify to the cobra-venom by C18 revert column on the HPLC chromatography system with the chromatographic condition: mobile phase 0.1% trifluoroacetic acid and methyl cyanide, the mobile speed 7ml / min, it dose gradient elution on the gradient range 0-80% with the time 0-120min, the test wave length 210nm and the separating temperature 20-28 deg.
Owner:SOUTHERN MEDICAL UNIVERSITY
Inactivation method for snake venom and inactivated snake venom prepared thereby
InactiveCN102241760ALow toxicityLess antigenPeptide preparation methodsAnimals/human peptidesAntigen epitopeNeutralising antibody
The invention relates to an inactivation method for snake venom. According to the method, snake venom is alkylated by a protein alkylating reagent, and therefore snake venom is inactivated. Preferably, alkylation is carried out under reversible denaturation conditions and finished after lucifugal culturing for 1 to 10 hours at a temperature of 20 to 40 DEG C; the protein alkylating reagent is iodoacetamide, iodacetic acid or iodoacetate; the alkylation is carried out in a buffer solution for snake venom; the concentration of snake venom is 0.01 to 100 mg / ml; and snake venom is venom of viper or pallas pit viper, cobra, long-noded pit viper and coral snakes. The invention also relates to inactivated snake venom prepared by the inactivation method. The method is novel and enables no obvious change of conformation of snake venom and retention of critical antigen epitope; therefore, when inactivated snake venom is immunized, high titer neutralizing antibodies can be generated, the amount of antigen for immunization is reduced, side reactions are avoided, rapid generation of high titer neutralizing antibodies is obtained, and the method is suitable for large scale popularization and application.
Owner:SHANGHAI SERUM BIOTECH
Anti-venomous-snake PLA2 protein antibodies and application thereof
InactiveCN110317270AImprove diagnostic efficiencyHigh affinityImmunoglobulins against animals/humansDisease diagnosisMonoclonal antibodyAntibody screening
The invention relates to anti-venomous-snake PLA2 protein antibodies and application thereof. The anti-venomous-snake PLA2 protein antibodies comprise monoclonal antibodies of tritoxomab-PLA2, protoxomab-PLA2, viptoxomab-PLA2, najatoxomab-PLA2 or glotoxomab-PLA2, and have high affinity and specificity. The monoclonal antibodies are prepared by adopting PLA2 proteins of the five kinds of venomous snakes as antigens for immunizing mice through a hybridoma fusion technology, antibody screening is conducted by taking the amino acid sequences of of two hypervariable regions in the PLA2 proteins asantigens, and finally, ten monoclonal antibodies of tritoxomab-PLA2, protoxomab-PLA2, viptoxomab-PLA2, najatoxomab-PLA2 and glotoxomab-PLA2 are obtained in total. One or a combination of more of the ten monoclonal antibodies can be used as a diagnostic test reagent for snake wounds. The anti-venomous-snake PLA2 protein antibodies can be prepared into the diagnostic reagent or kit for the snake wounds, and the diagnostic efficiency of the snake wounds is improved.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Agkistrodon acutus hemocoagulase gene and methods for preparing expression vector, host cell and recombinant protein thereof
InactiveCN102660565ASingle ingredientDefinite hemostatic effectHydrolasesFermentationEnzyme GeneArginine
An agkistrodon acutus hemocoagulase gene and methods for preparing an expression vector, a host cell and recombinant protein thereof belong to biomedicine. Hemocoagulase maturation protein is single-chain glycoprotein with the molecular weight of 36000+ / -3000Da and 6.59 isoelectric points, wherein the single-chain glycoprotein contains 236 amino acid residue, the total order of proteinogenic amino acid is SEQNO:1, and a gene for encoding the protein is formed by 783 nucleotides and has the order of SEQNO:1. The hemocoagulase has arginine esterase activity and fibrinogen hydrolytic activity and can preferentially degrade an A Alpha chain of fibrinogen. The agkistrodon acutus hemocoagulase gene can be applied to genetic engineering preparation. Methods utilizing the agkistrodon acutus hemocoagulase gene to construct the expression vector and utilizing the host cell to obtain recombinant hemocoagulase protein are provided, and a method for obtaining the agkistrodon acutus hemocoagulase gene in the molecular biology is restated.
Owner:郑颖 +1
A kit for detecting circulating antigens of Agkistrodon acutina using specific polyclonal antibodies
InactiveCN102288754AFacilitate early diagnosisAids in prognosisMaterial analysis by observing effect on chemical indicatorSerum immunoglobulinsNitrocellulosePentastomiasis
The invention provides a kit for detecting circulating antigens of Agkistrodon acutinae using specific polyantibody, comprising rabbit anti-Aag-IgG, enzyme-labeled antibody (HRP-Aag-IgG), nitrocellulose film, 1 % bovine serum albumin in PBST solution, PBST washing solution, substrate chromogenic solution, positive and negative control sample composition and polyethylene reaction plate. The invention also provides a preparation method of a kit for detecting circulating antigens of Agkistrodon acutina by using specific polyantibody. The invention adopts the polyclonal antibody to measure the circulating antigen, which is beneficial to making early diagnosis and helping to judge the prognosis. When the parasites in the body die, the circulating antigens disappear quickly, which can be used for efficacy assessment. The kit of the invention has high sensitivity and strong specificity, and can realize rapid diagnosis, drug screening and curative effect assessment of Agkistrodon acutinae ligamentiworm disease.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Agkistrodon acutus hemocoagulase-B
ActiveCN102925422AGood coagulation activityReduce bleedingPeptide/protein ingredientsEnzymesSerine proteinasesPurification methods
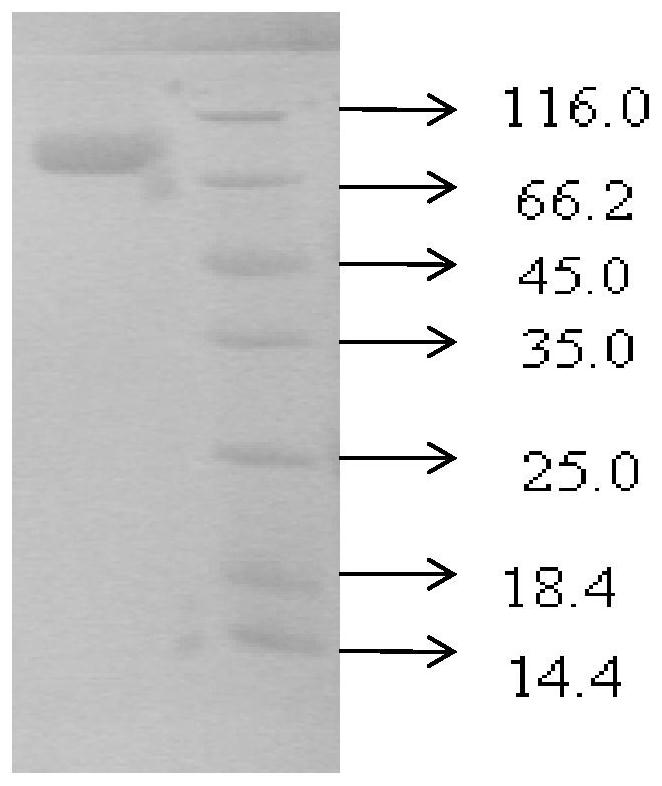
The invention provides agkistrodon acutus hemocoagulase-B which is high-activity hemocoagulase separated from agkistrodon acutus venom. The hemocoagulase is single-stranded glycoprotein comprising 236 amino acids, and has an amino acid sequence shown as SEQ ID No. (sequence identification number) 1. The strand comprises 6 pairs of disulfide bonds; molecular weight of SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) is approximately 35kD; the molecular weight of the hemocoagulase after being deglycosylated is 26116.7Da; and an isoelectric point (pI) is 6.0. A hemocoagulase molecule is modified by heterozygotic polysaccharides at an Asn<77, 100, 229> (asparagine) site. The hemocoagulase is serine proteinase. The invention also provides a separation and purification method of the hemocoagulase, which comprises the steps that undissolved substances are removed through pretreating; then two times of anion-exchange column chromatography and one time of Sephdex-G75 molecular sieve chromatography are conducted; an active elution peak is acquired; dialysis and lyophilization are conducted; and the high-purity agkistrodon acutus venom hemocoagulase is obtained. The specific activity of the agkistrodon acutus venom hemocoagulase is not less than 2000U / mg; the analysis purity of HPLC (high performance liquid chromatography) can reach above 95%; and the yield is 0.25-0.30% calculated according to the weight of an agkistrodon acutus venom raw material.
Owner:BEIJING KONRUNS PHARM CO LTD
Quantitative detection method of venom thrombin-like enzyme
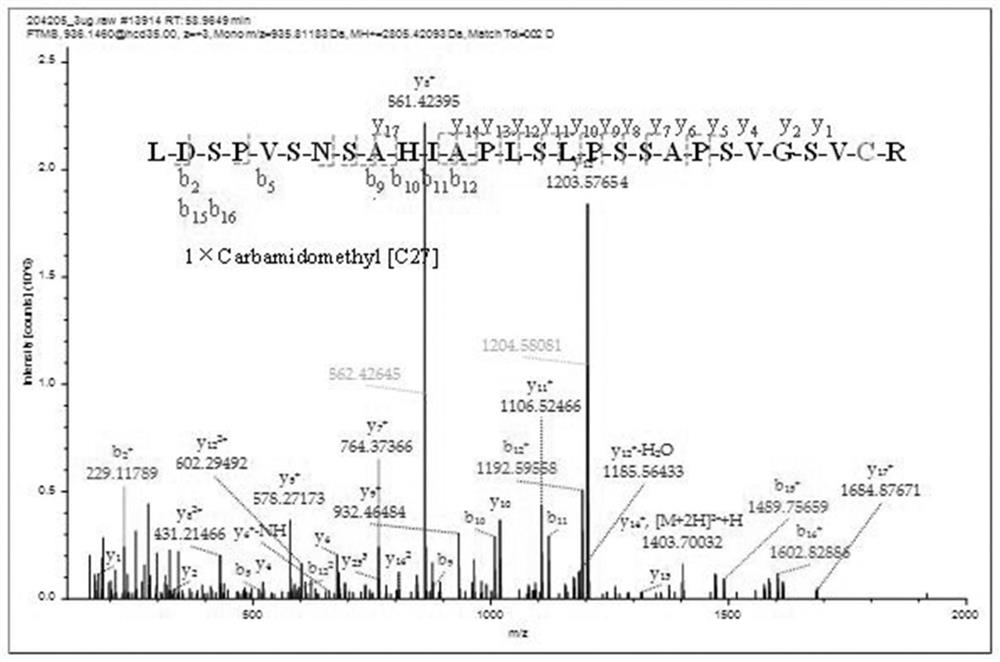
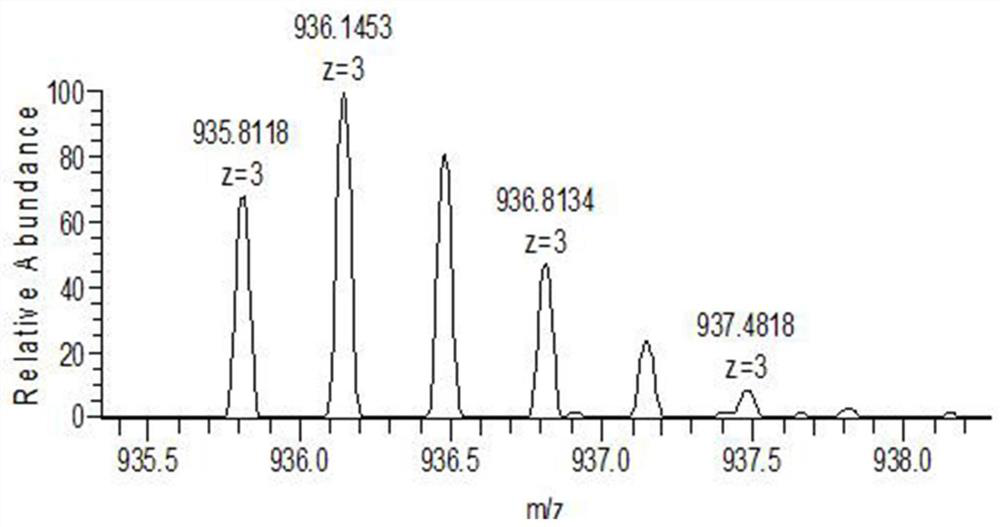
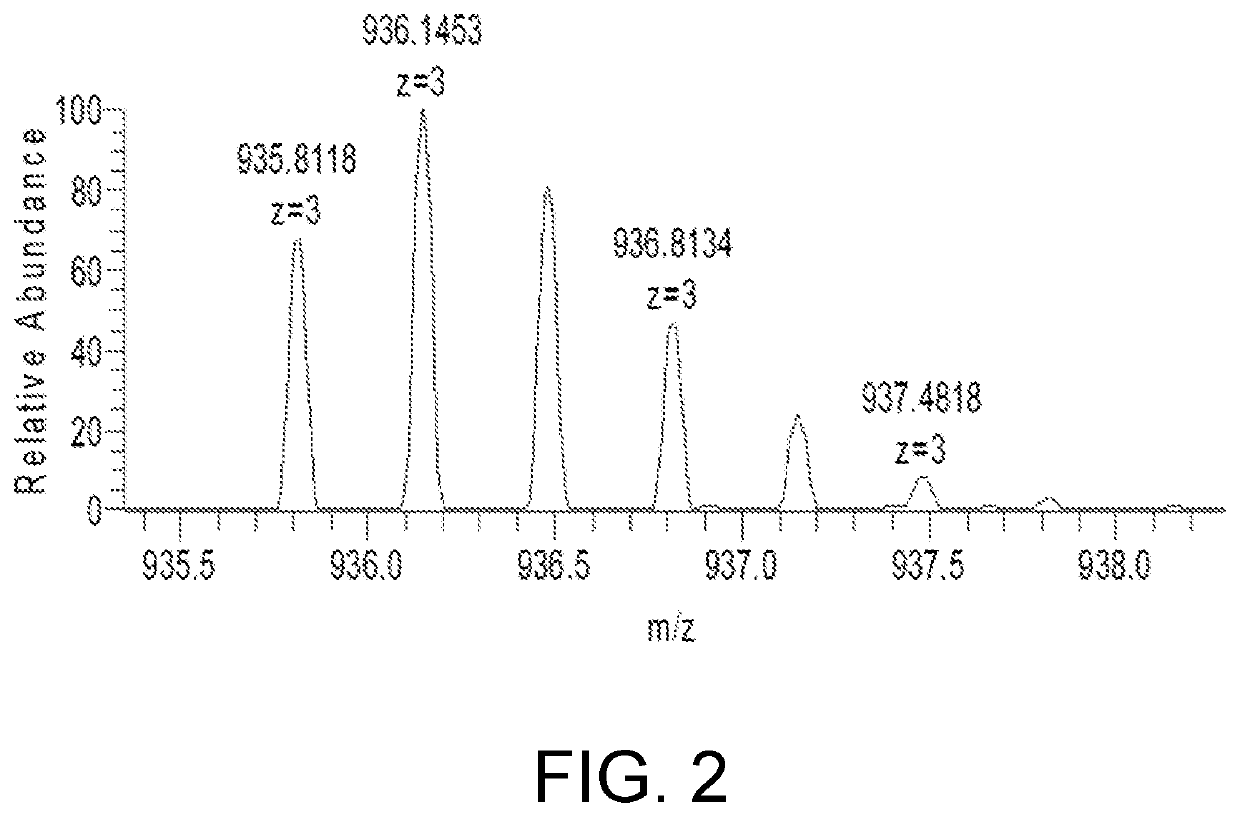
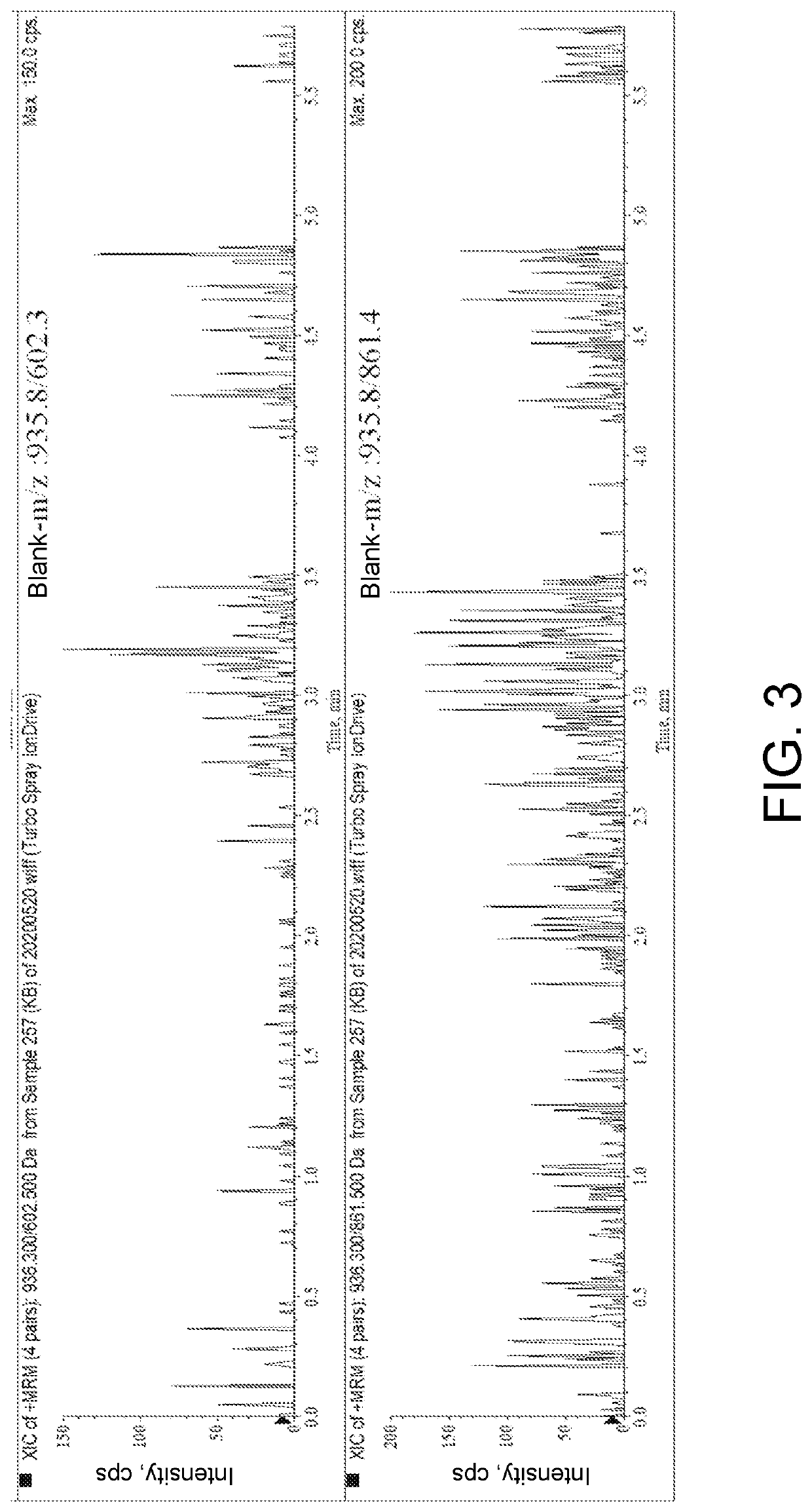
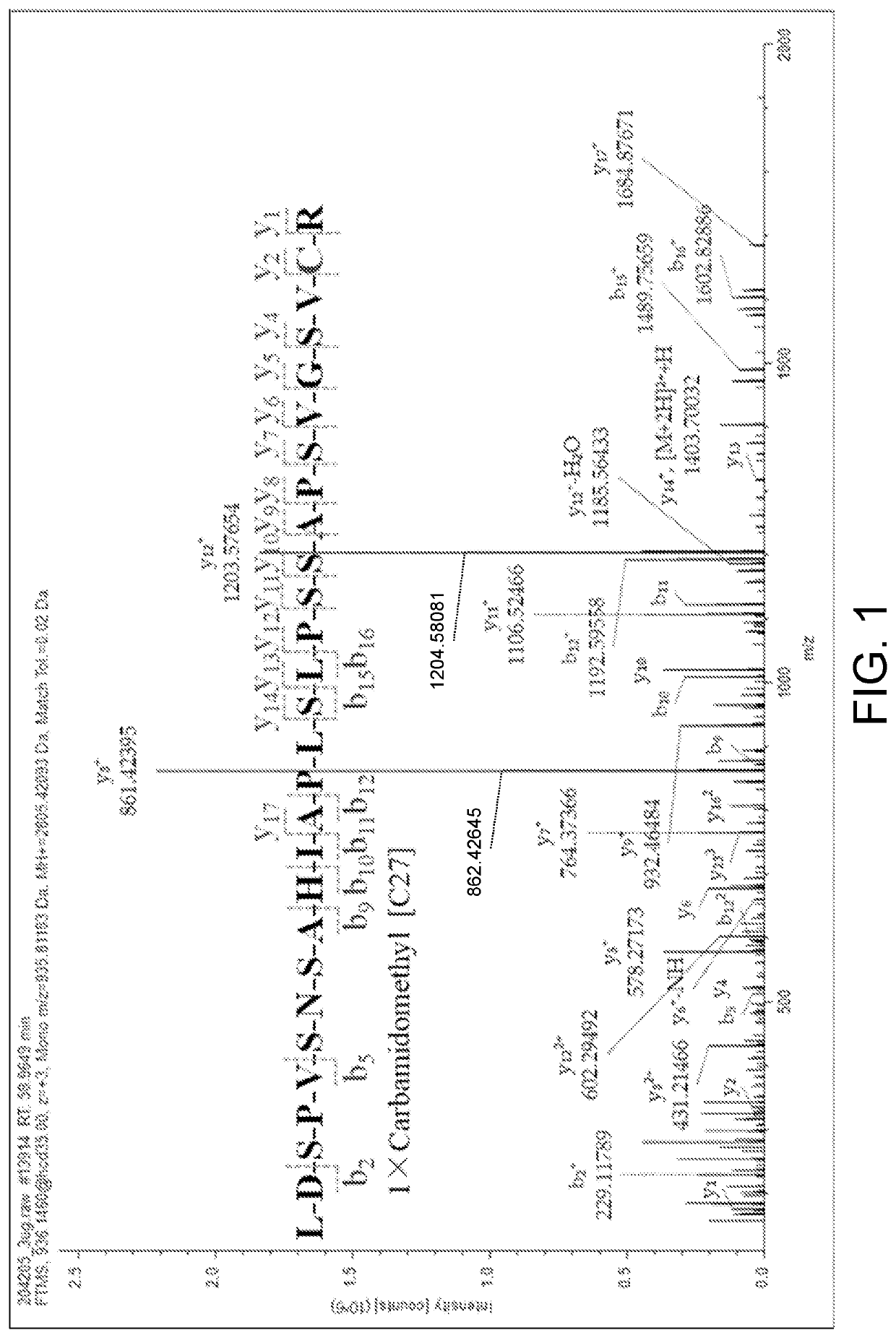
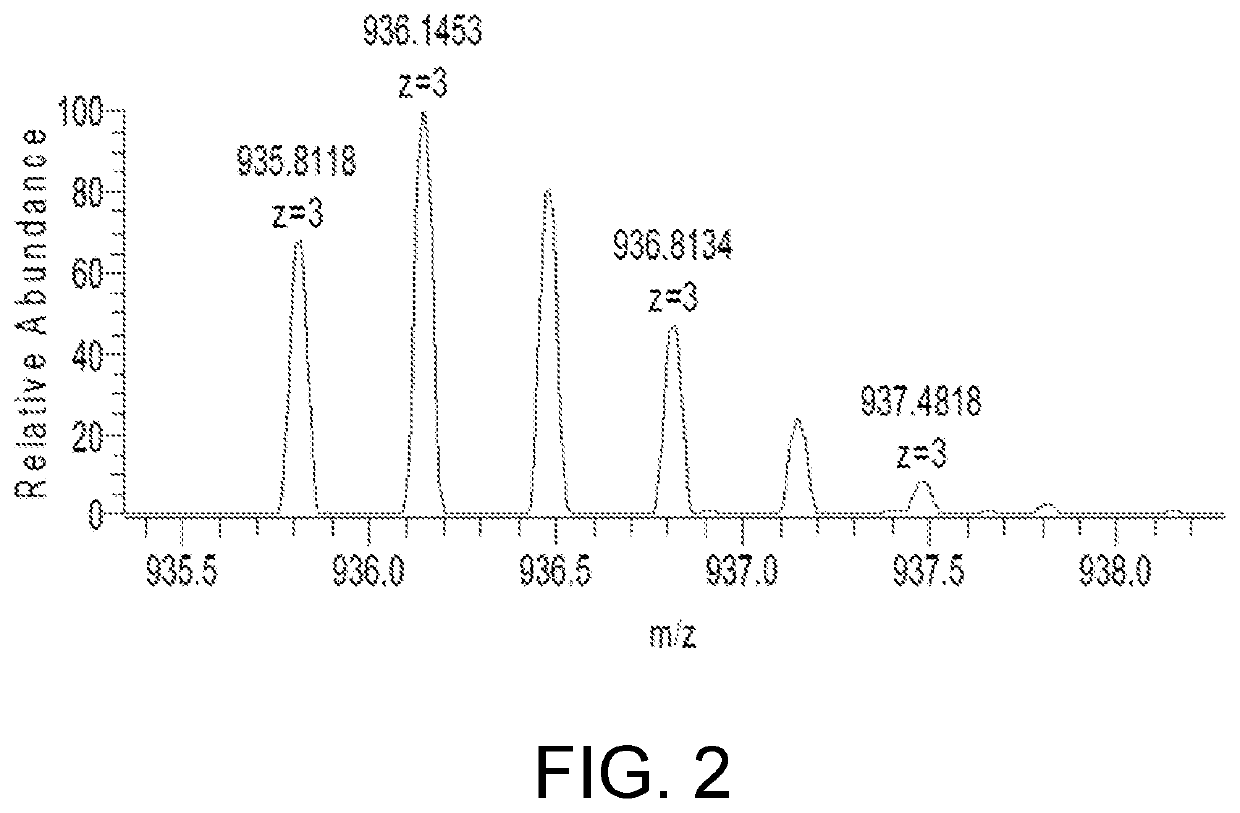
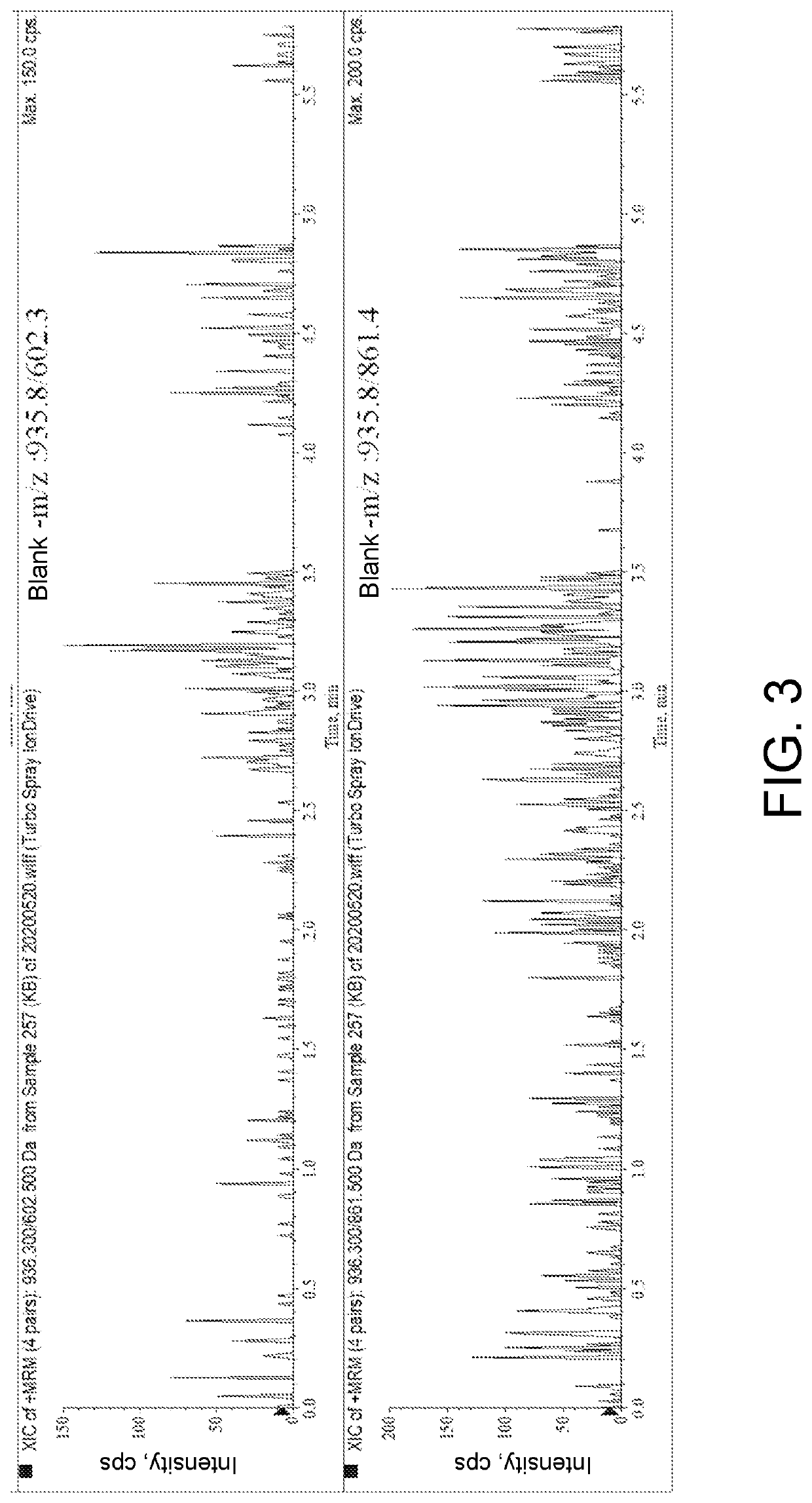
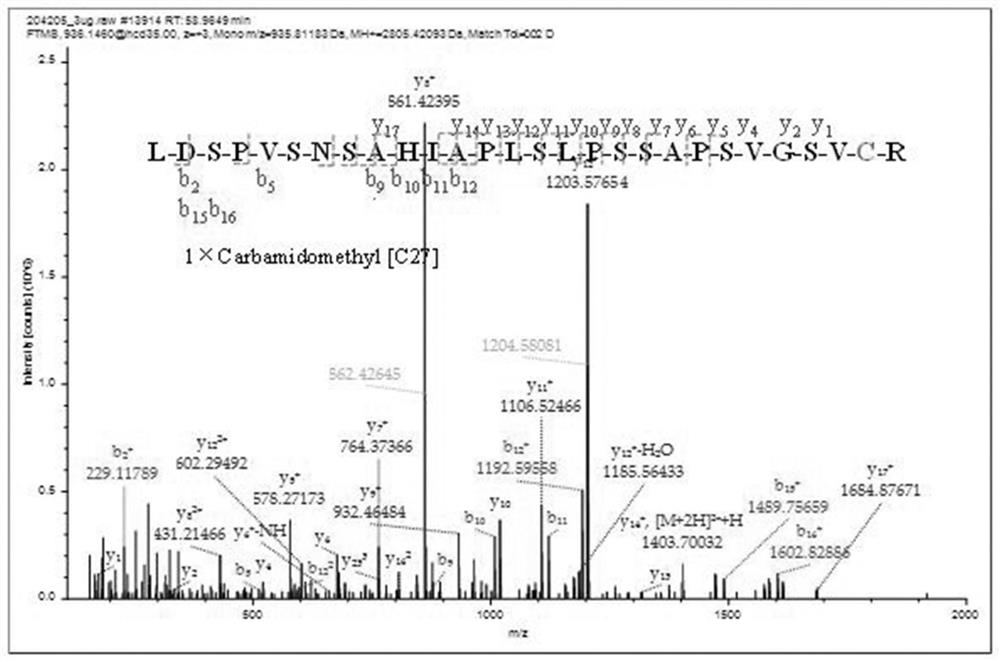
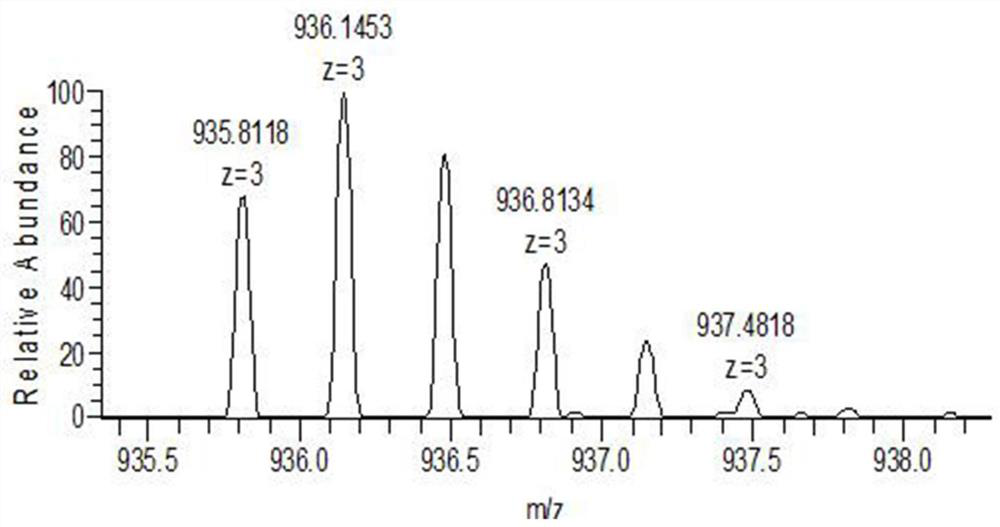
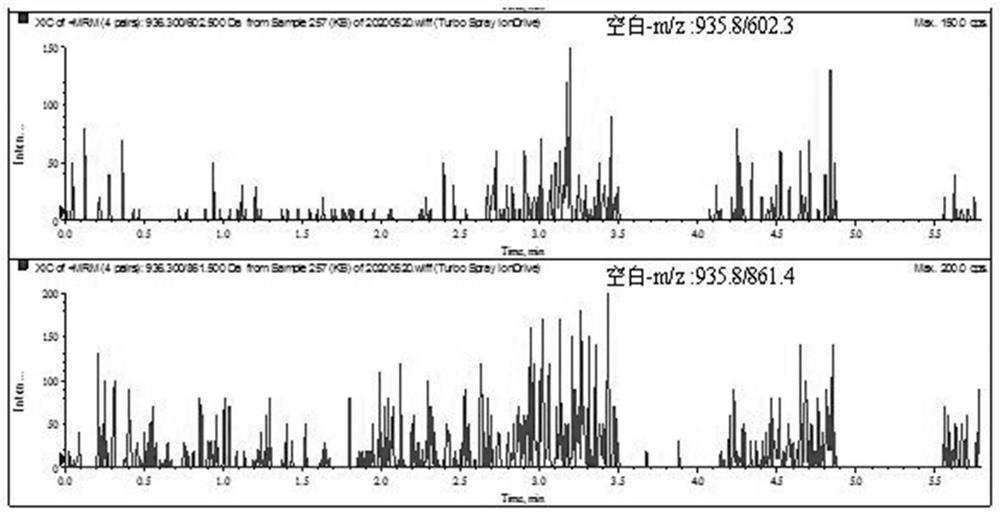
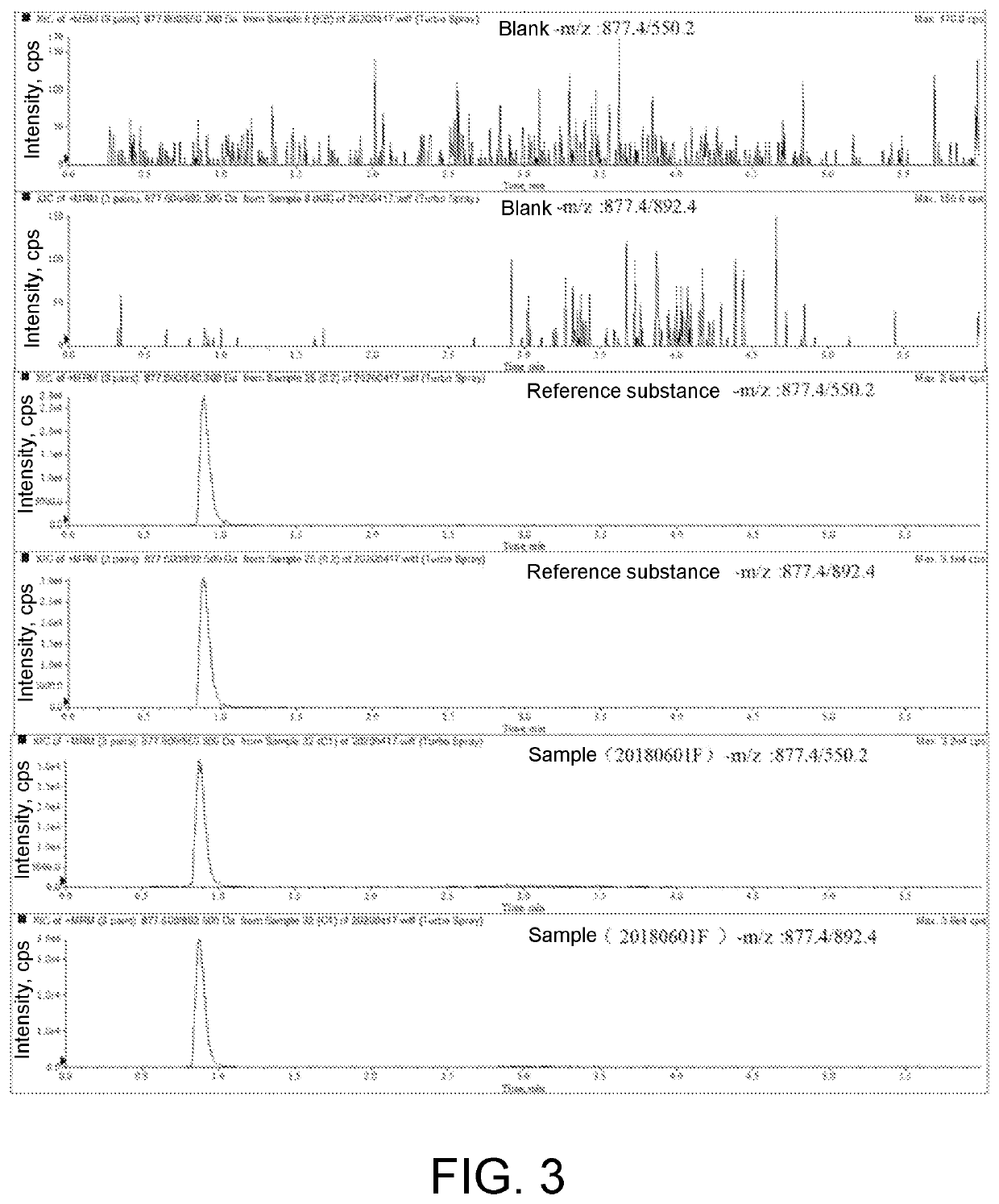
ActiveCN111896652AReduce distractionsQuantitatively accurateComponent separationMicrobiological testing/measurementMass spectrometricAmino acid
The invention relates to the technical field of chemical analysis and quantitative detection, in particular to a quantitative detection method of venom thrombin-like enzyme. The quantitative detectionmethod of the venom thrombin-like enzyme comprises the following steps: taking an agkistrodon halys venom thrombin-like enzyme characteristic polypeptide reference substance with an amino acid sequence of LDSPVSNSAHPLSLPSSAPSVGSVCR to prepare a series of concentration reference substance solutions; respectively adding the test solution, carrying out enzymolysis, and taking the supernatant after enzymolysis as a series of solutions to be detected; and respectively injecting a to-be-detected solution into a liquid chromatography-mass spectrometer, and selecting a qualitative ion pair and a quantitative ion pair to detect the content of the characteristic polypeptide in the to-be-detected solution. According to the method, the content of the thrombin-like enzyme in the agkistrodon halys venom and the extract thereof is determined by adopting a standard addition method, the method is simple, convenient and rapid, the matrix interference is less, the sensitivity is high, the quantificationis accurate, the blank of the quality standard of the agkistrodon halys venom and the extract thereof is filled, and the quality control level is improved.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
Fibrinolytic enzyme from gloydius intermedius venom and preparation method and application thereof
The invention provides a fibrinolytic enzyme from gloydius intermedius venom. The gene of the fibrinolytic enzyme is Gisp6, which has a nucleotide sequence as shown in SEQ ID No.1 of the sequence table and an amino acid sequence as shown in the SEQ ID No.2 of the sequence table. A preparation method of the fibrinolytic enzyme consists of three steps: a purification method of the gloydius intermedius venom fibrinolytic enzyme, purity identification and mass spectrometry analysis, and cloning of the gloydius intermedius venom fibrinolytic enzyme Gisp6 gene. The invention also provides application of the gloydius intermedius venom fibrinolytic enzyme in the establishment of a stable expression cell line in HEK293T cells and application of the fibrinolytic enzyme in thrombolytic drugs. By theadoption of the optimal salt concentration, the non-specific adsorption of components and fillers is reduced. By adopting the optimal pH value, peaks do not overlap, impurities are eliminated, and product purity is improved.
Owner:SHAANXI NORMAL UNIV
Agkistrodon acutus anticoagulant factor XI polypeptide and application thereof
ActiveCN113773377AStrong inhibitory activityProlonged thromboplastin timePeptide/protein ingredientsProtease inhibitorsAntithrombotic AgentAgkistrodon acutus
The invention belongs to the field of polypeptides in biochemistry, and relates to an agkistrodon acutus anticoagulant factor XI polypeptide and application thereof. According to the polypeptide, a Kunitz type polypeptide sequence is screened from an agkistrodon acutus snake venom transcriptome database, the Kunitz type polypeptide sequence is constructed and expressed to obtain the polypeptide with antithrombotic activity, and the polypeptide can be applied to preparation of antithrombotic drugs.
Owner:CHINA PHARM UNIV
Quantitative detection method for snake venom thrombin-like enzyme (SVTLE)
ActiveUS20220033873A1The process is convenient and fastLess interferenceComponent separationMicrobiological testing/measurementLiquid chromatography mass spectroscopyMass spectrometric
The present invention relates to the technical field of chemical analysis and quantitative detection, in particular to a quantitative detection method for snake venom thrombin-like enzyme (SVTLE) from Agkistrodon halys pallas. The quantitative detection method for the SVTLE includes the following steps of taking a reference substance of marker peptide for the SVTLE from Agkistrodon halys pallas with an amino acid sequence of LDSPVSNSAHIAPLSLPSSAPSVGSVCR, and preparing a series of reference solutions with different concentrations; adding the reference solutions in test solutions respectively for enzymolysis, and then taking a supernatant after enzymolysis as a series of solutions to be detected; and adding the solutions to be detected in a liquid chromatogram-mass spectrometer, and then selecting a qualitative ion pair and a quantitative ion pair to detect contents of marker peptide in the solutions to be detected.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
Agkistrodon halys venom identification method applying mass spectrometry and application of method
PendingCN111458420AQuality is easy to controlStrong targetingComponent separationMaterial analysis by electric/magnetic meansMass Spectrometry-Mass SpectrometryDiagnosis laboratory
The invention discloses a method for identifying agkistrodon halys venom by applying mass spectrometry. The method comprises the following steps of: (1) dissolving agkistrodon halys venom to prepare aprotein solution; (2) carrying out SDS-PAGE analysis; and (3) carrying out LC-MS / MS mass spectrometry to carry out agkistrodon halys venom component identification. In addition, the invention furtherdiscloses application of the identification method in the preparation of products for identifying the agkistrodon halys venom and application in the preparation of agkistrodon halys venom resistant serum for treating agkistrodon halys venom poisoning. According to the invention, the agkistrodon halys venom can be identified in a laboratory; agkistrodon halys venom is more accurately subjected toquality control, so that specific drug anti-agkistrodon halys poison serum for treating agkistrodon halys venom poisoning is produced; and the method is very important for ensuring the safety, effectiveness and quality controllability of anti-agkistrodon halys venom serum products and is of great significance for reference of the diagnosis and legal medical expert identification of the types of venom caused by snake venom poisoning. The method has the advantages of high detection sensitivity, high correct detection rate, no cross reaction and simple operation process.
Owner:SHANGHAI SERUM BIOTECH
Marker peptide of snake venom thrombin-like enzymes (SVTLEs) from agkistrodon halys pallas and application thereof
ActiveUS11467141B2The process is convenient and fastQuantitative precisionComponent separationEnzymesMass analyzerMass
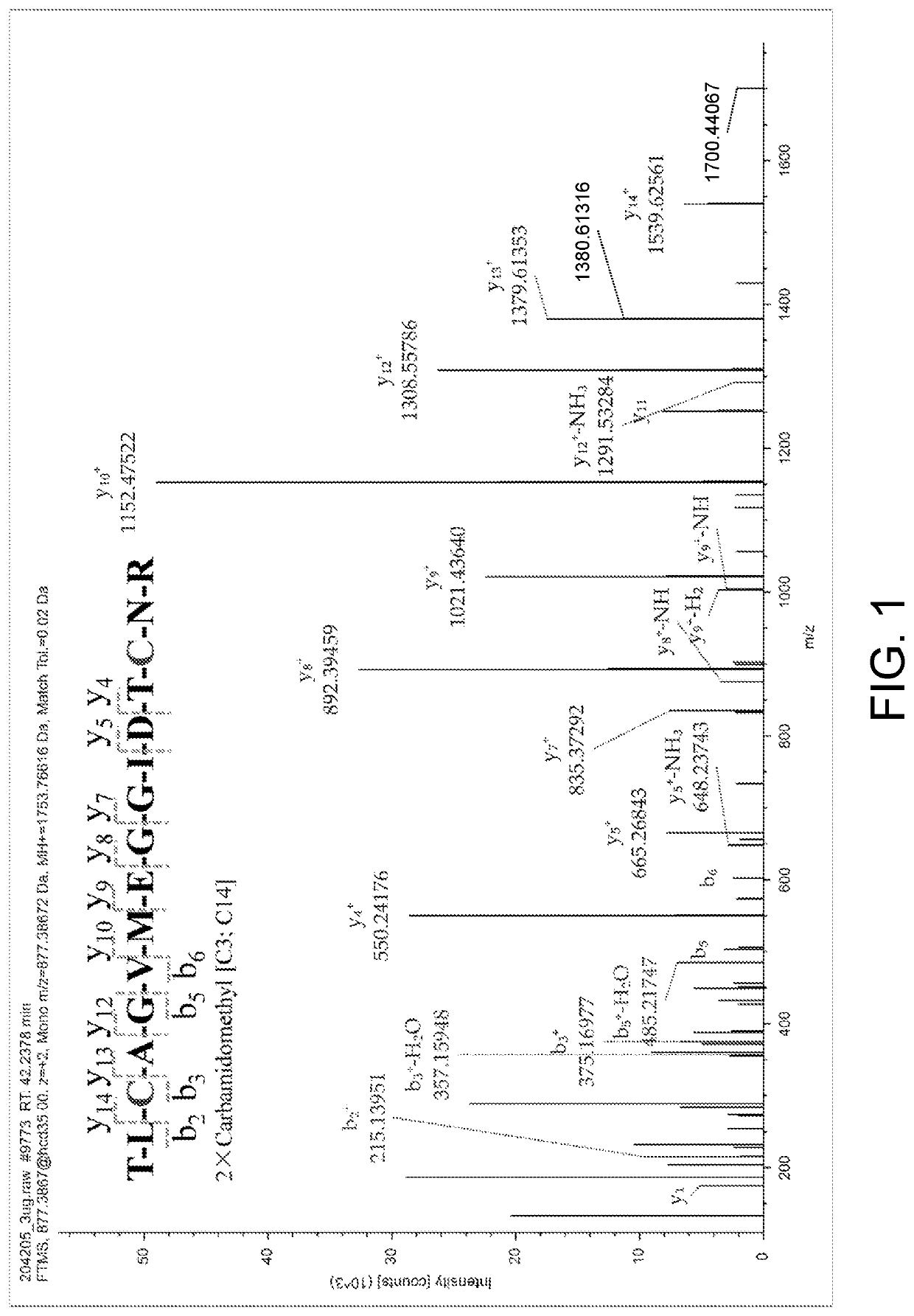
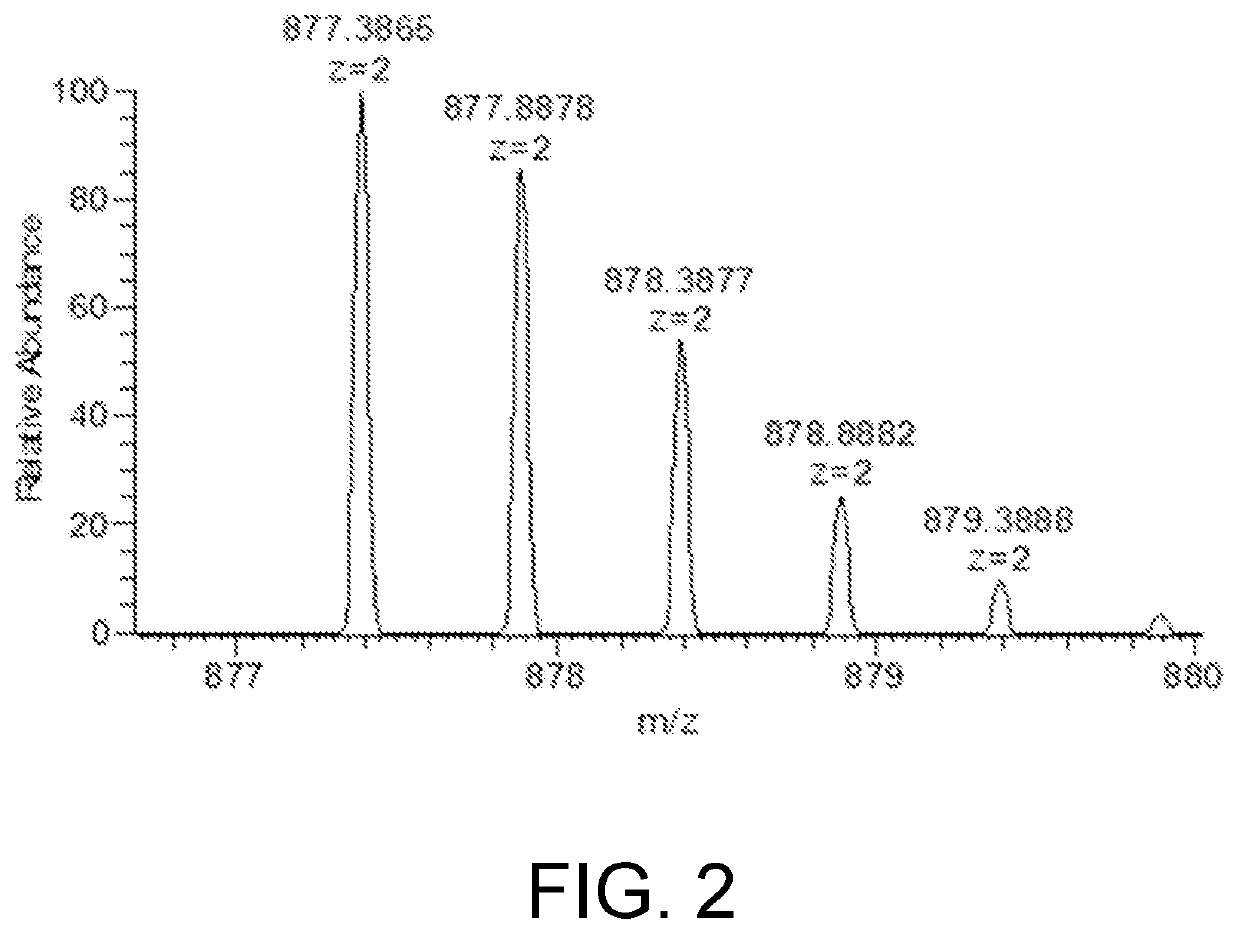
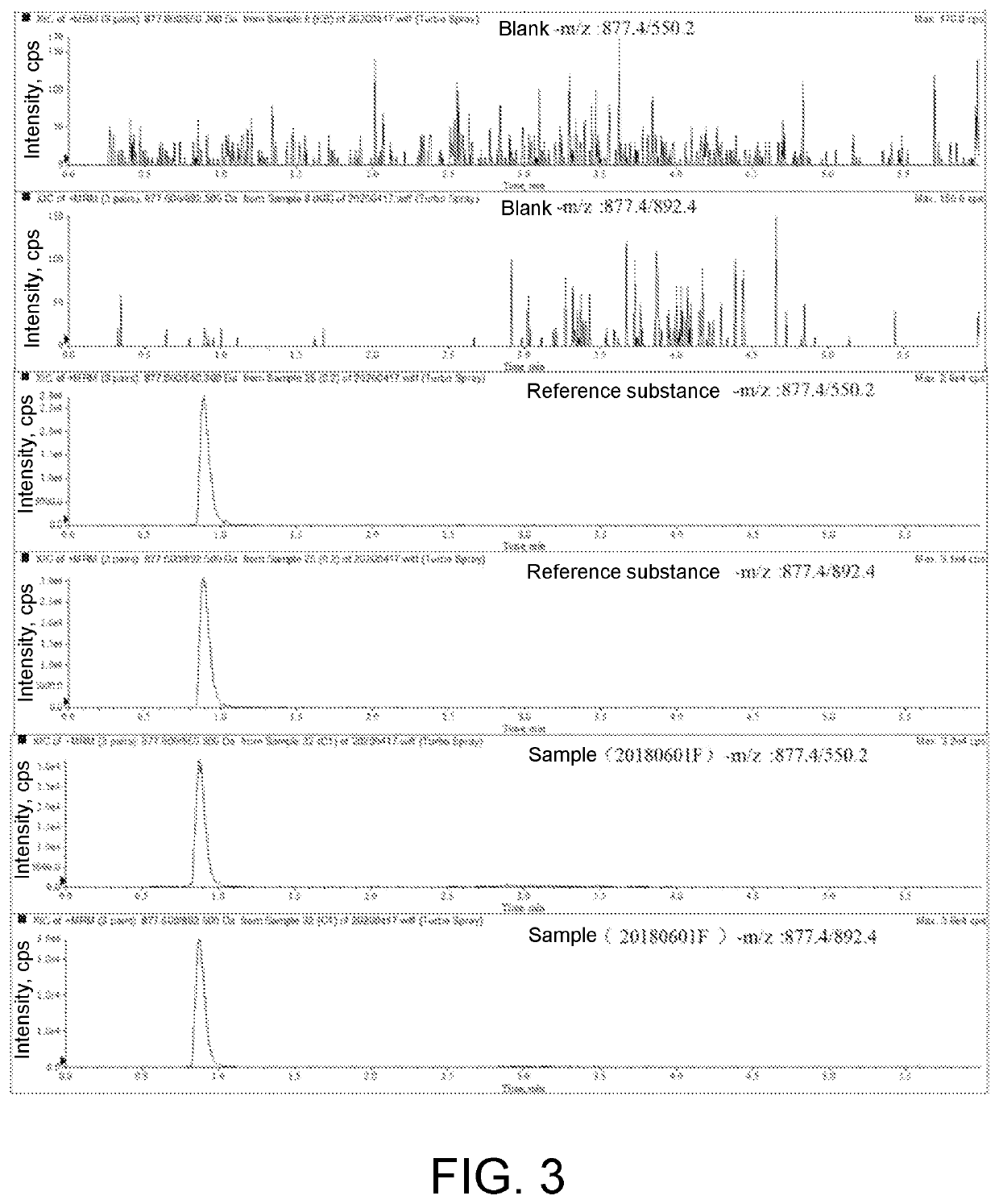
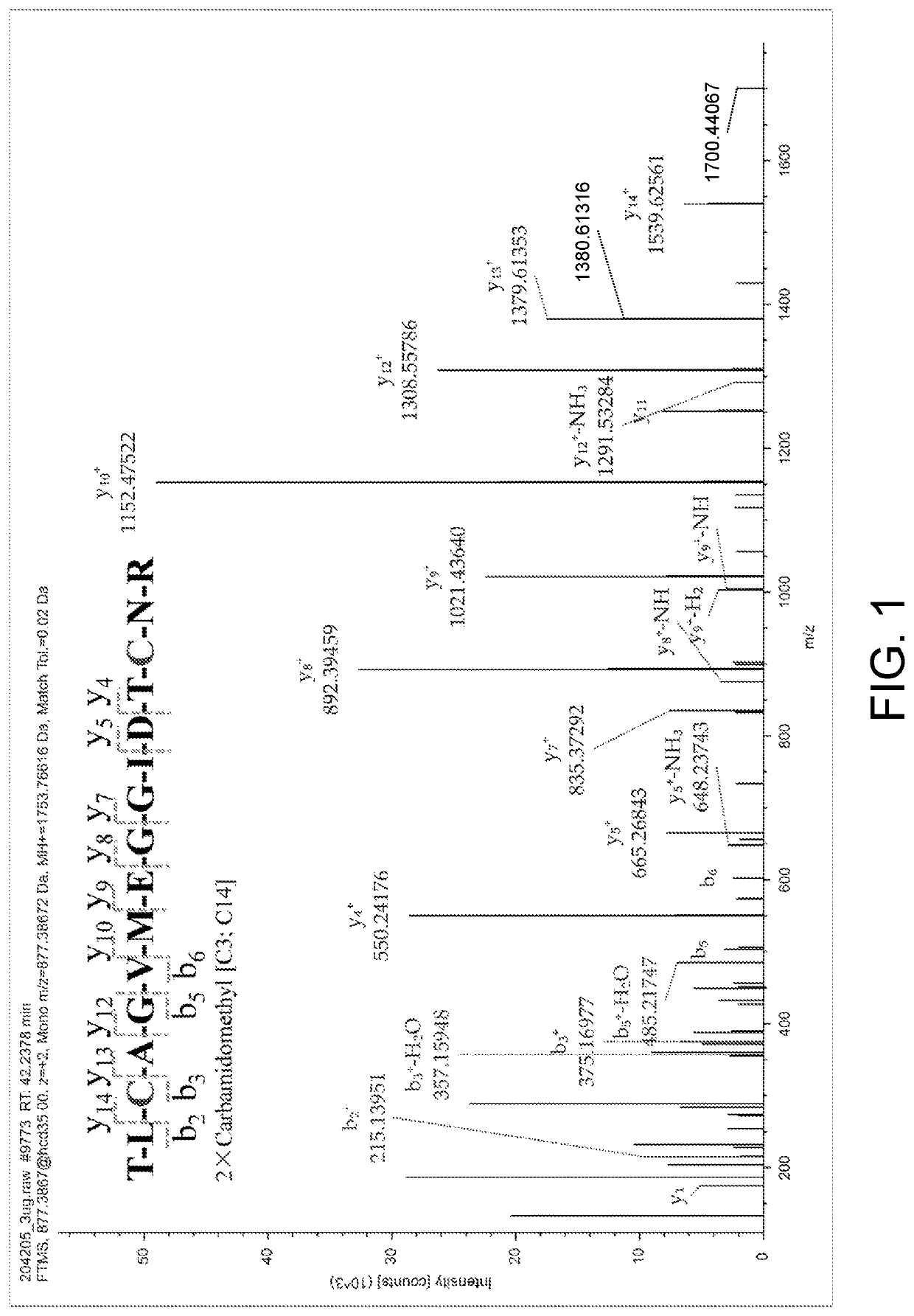
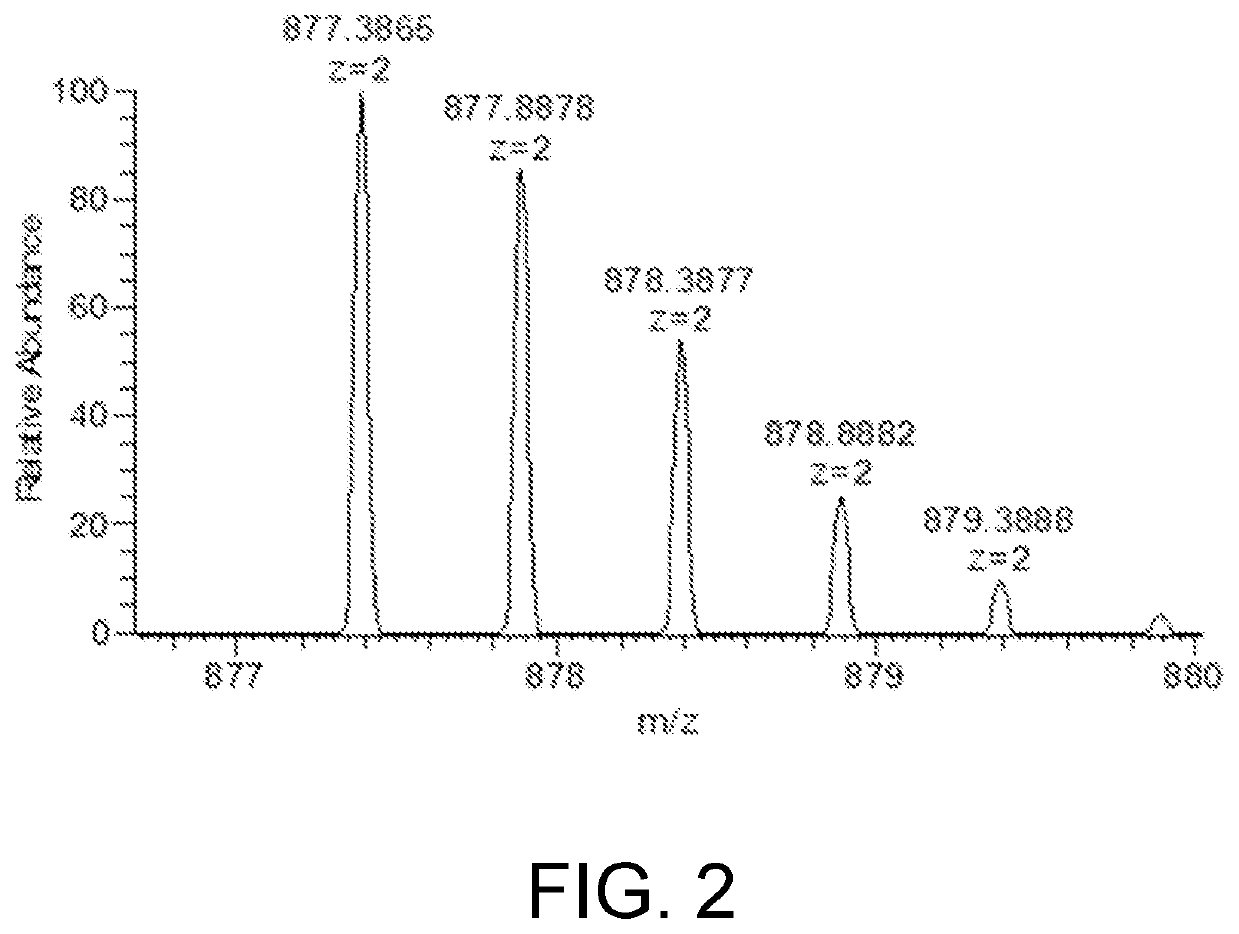
The present invention relates to the field of chemical analysis detection and application, in particular to a marker peptide of snake venom thrombin-like enzymes (SVTLEs) from Agkistrodon Halys Pallas and an application thereof. The amino acid sequence of the marker peptide of snake venom thrombin-like enzymes (SVTLEs) from Agkistrodon Halys Pallas is TLCAGVMEGGIDTCNR. Characterizing the source of species and a content of the SVTLEs in a to-be-detected sample by using the marker peptide includes the following steps of: pretreating the to-be-detected sample by trypsin through enzymolysis, and taking a supernatant of an enzymolysis liquid as a test solution; and injecting the test solution and a reference solution into a liquid chromatography-mass spectrometer, and selecting a qualitative ion pair and a quantitative ion pair for detecting the source of species and a content of the SVTLEs in the to-be-detected sample.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
Snake venom thrombin-like enzyme marker peptide of agkistrodon halys pallas and its application in the species identification of hemocoagulase for injection
PendingUS20220034853A1Strong specificityThe process is convenient and fastComponent separationMicrobiological testing/measurementHydrolysisMass spectrometric
The present invention provides a snake venom thrombin marker peptide of Agkistrodon Halys Pallas and an application of the snake venom thrombin-like enzyme in identifying species of Hemocoagulase for Injection. The application includes the following steps of: dissolving a to-be-detected sample and a reference substance of the marker peptide respectively to prepare a test solution and a reference solution, and conducting alkylation reduction on the test solution and the reference solution with dithiothreitol and iodoacetamide; after diluting products with an ammonium bicarbonate solution, adding enzyme for hydrolysis; and after enzymolysis is finished, conducting centrifugation at a high speed, and injecting a supernatan into a liquid chromatography-mass spectrometer for analysis. This method is simple, convenient and rapid, is strong in specificity, fills the gap in identifying the source of species of the snake venom thrombin-like enzyme of Agkistrodon Halys Pallas and improves the quality control level.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
Anti-inflammatory polypeptide DAvp-1 in snake venom and application of anti-inflammatory polypeptide DAvp-1
ActiveCN113388020AHas the effect of antagonizing TNF-αPeptide/protein ingredientsAntipyreticDeinagkistrodon acutusPeptide sequence
The invention belongs to the field of molecular biology and particularly relates to an anti-inflammatory polypeptide DAvp-1 in snake venom and application of the anti-inflammatory polypeptide DAvp-1. The invention specifically provides an anti-inflammatory polypeptide DAvp-1 with a polypeptide sequence as shown in SEQ ID NO: 2. According to the anti-inflammatory polypeptide DAvp-1 and the application thereof, a brand-new polypeptide DAvp-1 capable of being combined with TNFR1 is successfully screened from a phage library of venom of deinagkistrodon acutus. The polypeptide DAvp-1 has a potential effect of antagonizing TNF-alpha and is an excellent candidate for research and development of polypeptide anti-inflammatory drugs.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Gene sequence and amino acid sequence of snake venom plasmin of agkistrodon blomhoffii ussurensis
InactiveCN102719461AExcellent activityExcellent curative effectEnzymesFermentationForward primerTotal rna
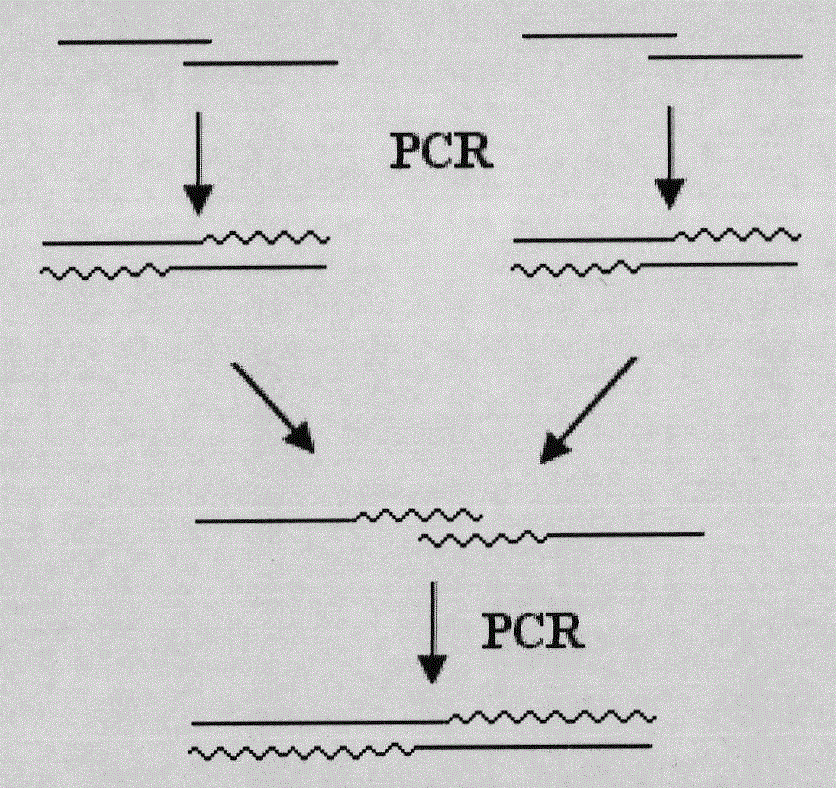
Plasmin is obtained from agkistrodon blomhoffii ussurensis by isolation and purification. Ten amino acid sequence at N terminal of the plasmin are measured, according to which forward primer is designed; and reverse primer is designed according to a region, wherein the region is much higher similar to the non-coding region at 3' terminal of the snake venom plasmin of other vipers. Total RNA is extracted from a poison gland; gene is amplified from the RNA by PCR. The result of complete sequence determination after cloning indicates that c DNA of the maturation protein codes 708 nucleotides, that is, 236 amino acids. The successful clone of the gene and the determination of the gene sequence create favorable conditions for the development of gene engineering products.
Owner:CHINA AGRI UNIV +1
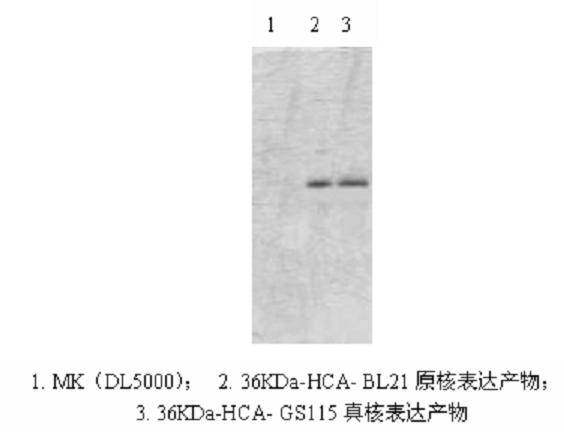
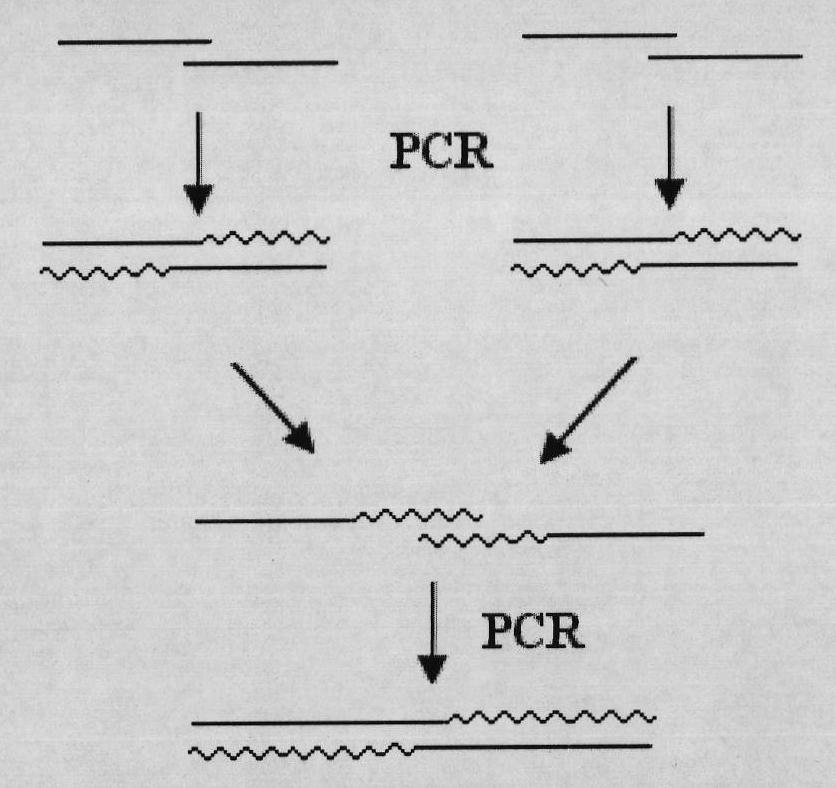
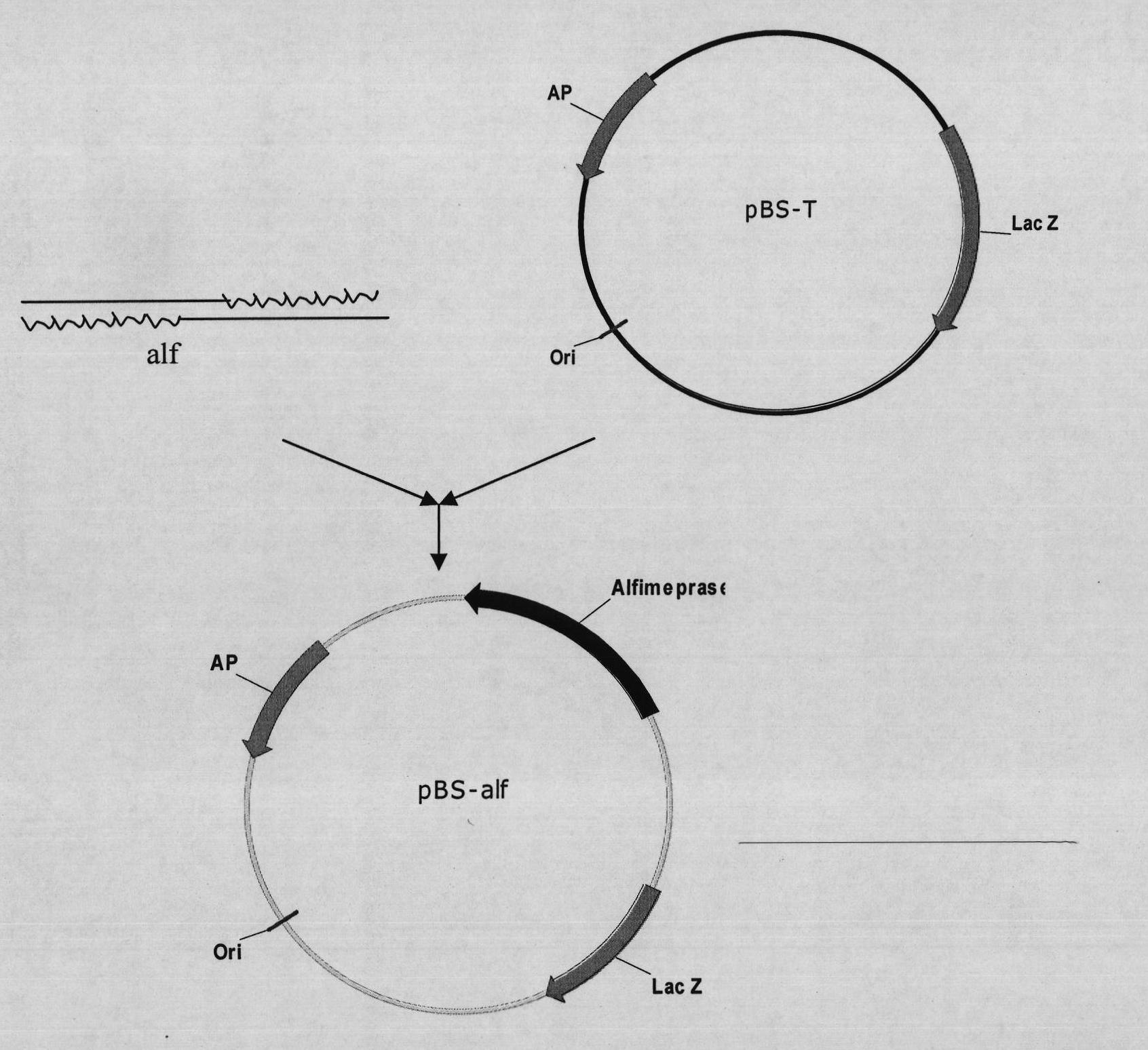
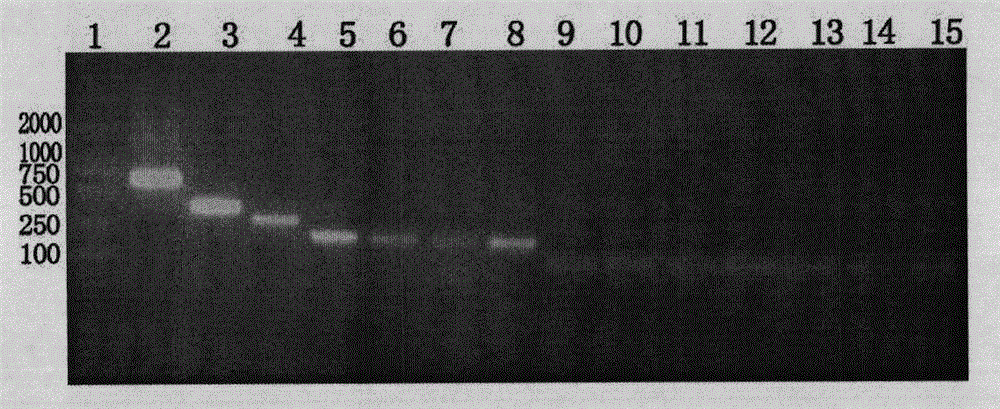
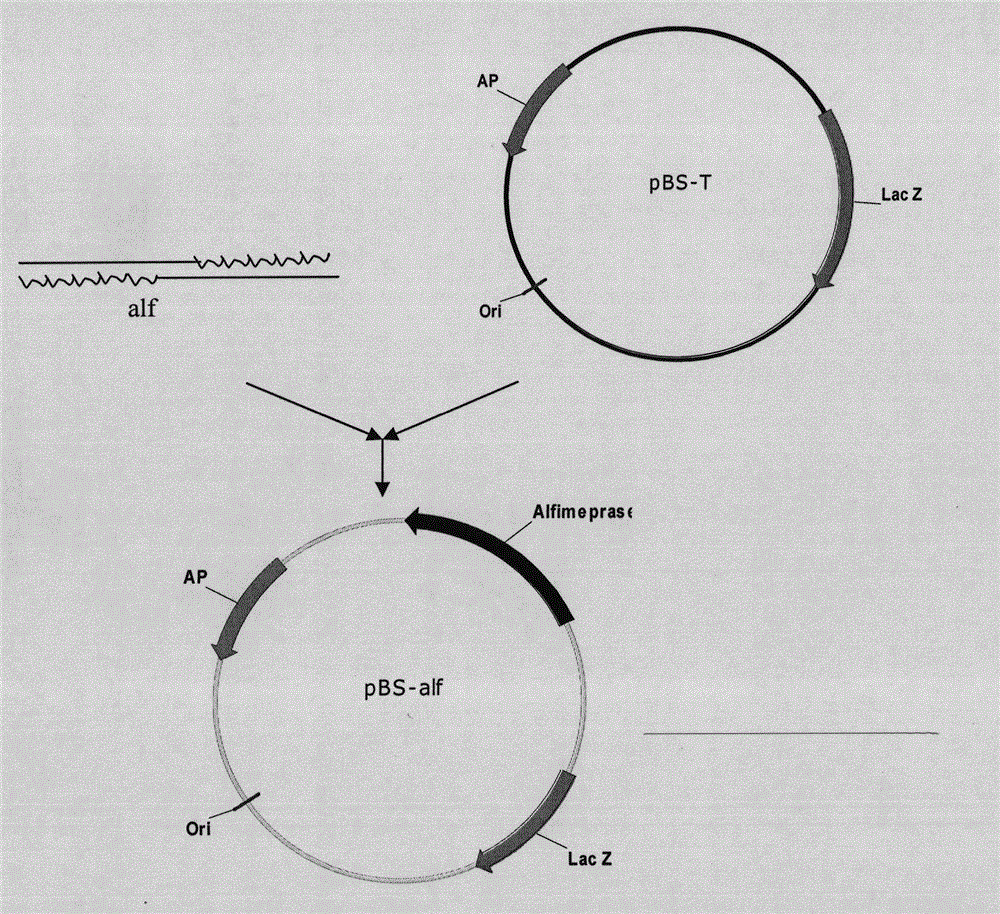
Mutant Alfimeprase engineering strain, and preparation method and applications thereof
The invention relates to a preparation method of a recombinant viper venom protein. A mutant Alfimeprase gene of artificially synthesized Agkistrodon contortrix is utilized, and the gene has the nucleotide sequence shown as SEQ ID No.2. The gene is linked to yeast expression plasmids pPIC9K to construct a high efficiency expression vector pPIC9K-His-Alf, a Pichia yeast strain GS115 is introduced, and the vector is named as FY-Alf. Induced expression is carried out with methanol, the expressed recombinant protein is purified and then subjected to activity verification by a fibrin plate assay, and the result shows that the recombinant protein Alfimeprase expressed by the yeast strain GS115 containing pPIC9K-His-Alf plasmids has high fibrinolytic activity.
Owner:ANHUI FENGYUAN PHARM CO LTD
Agkistrodon acutus hemocoagulase-B
ActiveCN102925422BGood coagulation activityReduce bleedingPeptide/protein ingredientsEnzymesSerine proteinasesPurification methods
The present invention provides a Agkistrodon haemagglutinase-B, which is a highly active hemocoagulase isolated from Agkistrodon akistros venom, and the enzyme is a single-chain glycoprotein composed of 236 amino acids. It has the amino acid sequence shown in SEQ ID No.1. The chain contains 6 pairs of disulfide bonds; the SDS-PAGE molecular weight is about 35kD, and the molecular weight of the deglycosylated enzyme is 26116.7Da; the isoelectric point pI is 6.0. The enzyme molecule has hybrid polysaccharide modifications at Asn77, 100, and 229 positions. The enzyme is a serine protease. The present invention also provides a separation and purification method for the enzyme, which includes removing insoluble matter through pretreatment, and then passing through two anion exchange column chromatography and one Sephdex-G75 molecular sieve chromatography to collect the active elution peak, dialysis, freeze-drying, That is, high-purity snake venom hemagglutinin. Its specific activity is not less than 2000U / mg, its HPLC analysis purity can reach more than 95%, and its yield is 0.25%-0.30% based on the weight of snake venom raw materials.
Owner:BEIJING KONRUNS PHARM CO LTD
A kind of quantitative detection method of snake venom thrombin
ActiveCN111896652BReduce distractionsQuantitatively accurateComponent separationMicrobiological testing/measurementMass spectrometricAmino acid
The invention relates to the technical field of chemical analysis quantitative detection, in particular to a quantitative detection method of snake venom thrombin. The quantitative detection method of the above-mentioned snake venom-like thrombin is as follows: the amino acid sequence is LDSPVSNSAHIAPLSLPSSSAPSVGSVCR the characteristic polypeptide reference substance of Agkistrodon halys venom, and a series of concentration reference substance solutions are prepared; The decomposed supernatant is used as a series of test solutions; the test solutions are respectively injected into the liquid chromatography-mass spectrometer, and the qualitative and quantitative ion pairs are selected to detect the characteristic polypeptide content in the test solution. The present invention adopts the standard addition method to measure the content of thrombin-like enzyme in Agkistrodon venom and its extract, the method is simple and fast, has less matrix interference, high sensitivity and accurate quantification, and fills the gap in the quality standard of Agkistrodon venom and its extract , Improved quality control level.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
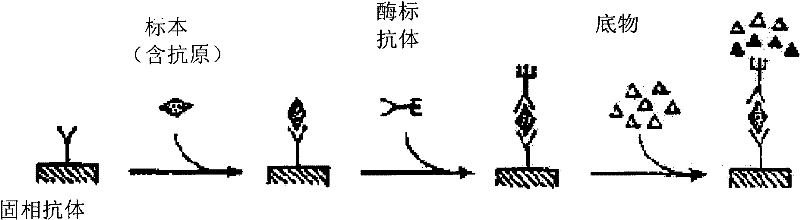
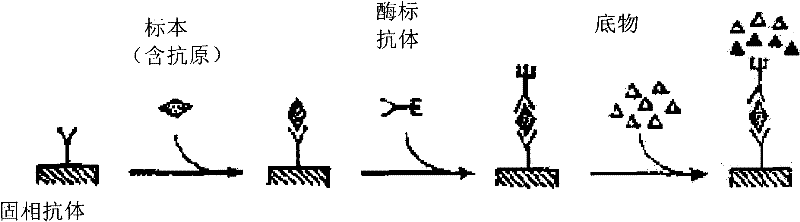
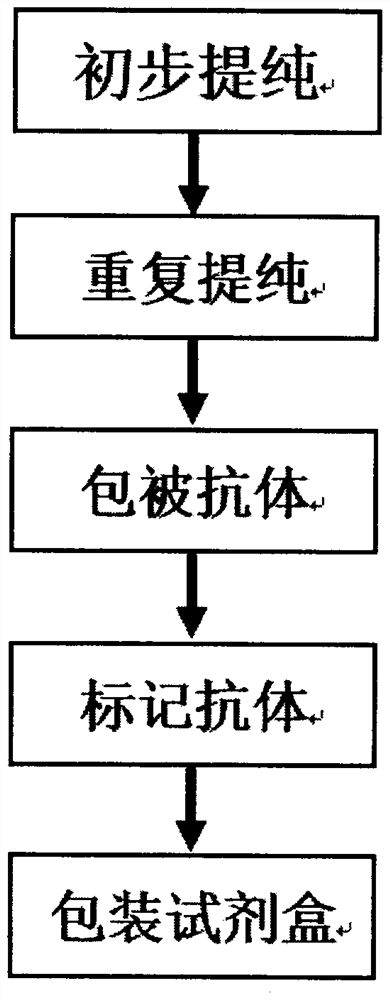
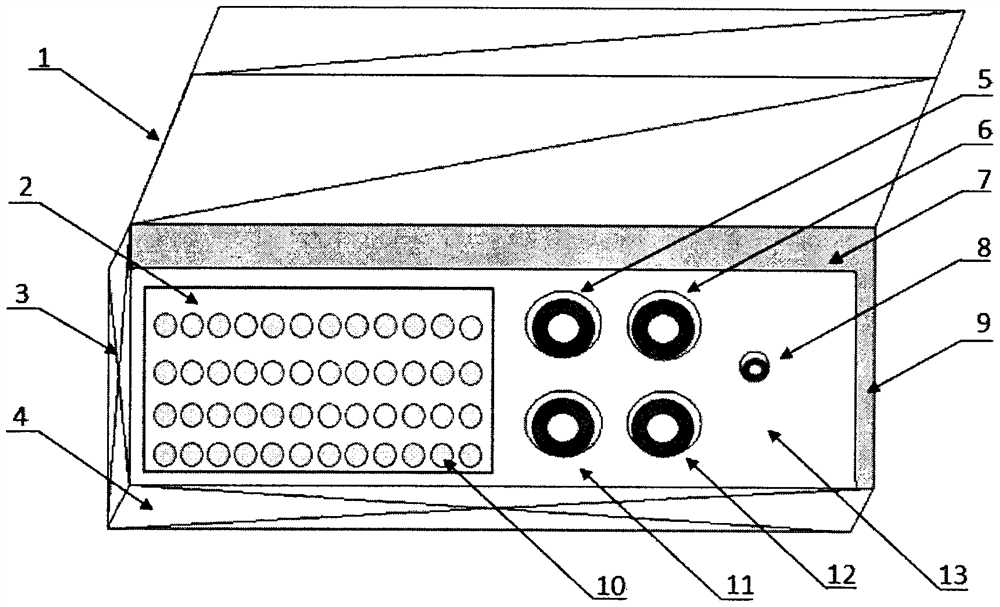
Preparation method of agkistrodon acutus venom detection kit based on improved double-antibody sandwich ELISA method
The invention discloses a preparation method of an agkistrodon acutus venom detection kit based on an improved double-antibody sandwich ELISA (Enzyme-Linked Immunosorbent Assay) method, which comprises the following steps: step 1, primary purification: primarily purifying horse anti-agkistrodon acutus IgG in horse anti-agkistrodon acutus plasma by adopting a saturated ammonium sulfate method; 2, repeatedly purifying, further performing chromatographic purification on the horse anti-agkistrodon acutus IgG by adopting affinity chromatography, collecting an antibody corresponding to a target peak, and performing column chromatography purification again to obtain relatively pure horse anti-agkistrodon acutus IgG; 3, coating an antibody: taking a part of the antibody purified in the step 2 as a coating antibody, and coating the coating antibody in a 48-pore plate; step 4, labeling an antibody, taking a part of the antibody purified in the step 2 to react with horse radish peroxidase (HRP) to obtain a horse radish peroxidase labeled antibody (HRP-IgG), and taking the HRP-IgG as a sandwich antibody; and 5, packaging the kit, sealing the 48-pore plate coated in the step 3 in vacuum, and putting the 48-pore plate and the sandwich antibody in the step 4 into the kit for later use, so that the kit is prepared and can be used for detecting whether human or animal serum contains agkistrodon acutus toxin or not.
Owner:宁宗 +2
Method for purifying hemagglutinin of spearhead agkistrodon halys
PendingCN114686463AShort purification cycleReduce risk of exposurePeptide/protein ingredientsBlood disorderHemagglutininBenzamidine
The invention provides a method for purifying agkistrodon spearhead hemocoagulase, which comprises the following steps: pretreating agkistrodon spearhead venom, and sequentially carrying out benzamidine agarose gel affinity chromatography, cation exchange chromatography and hydrophobic chromatography to obtain the agkistrodon spearhead hemocoagulase. According to the method provided by the invention, the process steps are further simplified, the purification period of the hemocoagulase is greatly shortened, the process environment exposure risk is reduced, the process stability is high, the product quality consistency is good, and the clinical medication safety is facilitated.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Agkistrodon blomhoffii ussurensis defibrase HPLC detection method and application
PendingCN113237987AAchieve separationMeet the quality standard requirementsComponent separationPerfluoroacetic AcidSilica gel
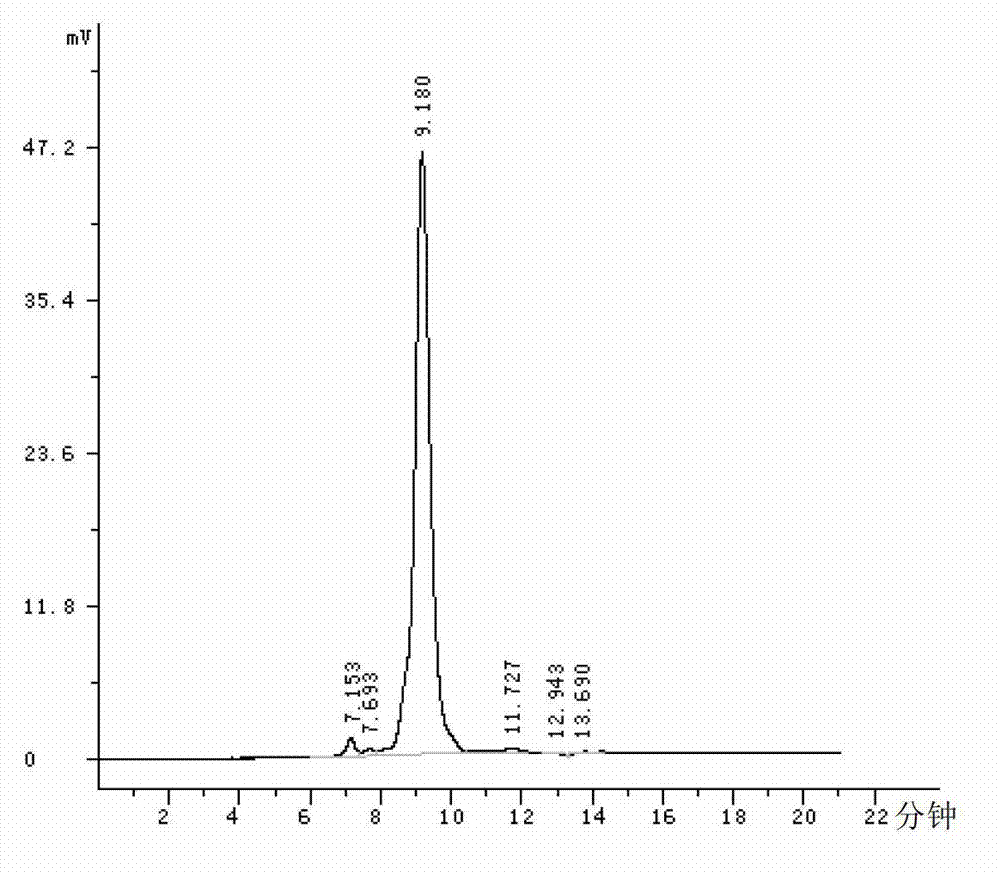
The invention relates to the technical field of biochemical detection, and particularly discloses an agkistrodon blomhoffii ussurensis defibrase HPLC detection method and application thereof. According to the HPLC detection method, a mobile phase A, a mobile phase B and a mobile phase C are adopted for elution; the mobile phase A is a 20-25 mM aqueous solution of sodium dihydrogen phosphate, and the pH value of the aqueous solution of sodium dihydrogen phosphate is 5.8-7.0; the mobile phase B is acetonitrile; and the mobile phase C is an aqueous solution of trifluoroacetic acid. Octadecylsilane chemically bonded silica is used as a filling agent in a reversed-phase column, and gradient elution is carried out. By establishing a purity determination method which is simple, convenient and sensitive and has good specificity, accuracy and durability, the quality of the agkistrodon blomhoffii ussurensis defibrase can be effectively controlled, and the quality of the agkistrodon blomhoffii ussurensis defibrase is stable, controllable, efficient and safe. Meanwhile, the method can also be used for identifying defibrases from different snake venom sources, and lays a foundation for improving quality standards of defibrase raw materials and preparations thereof.
Owner:BEIJING SAISHENG PHARMA
Traditional Chinese medicine composition for resisting agkistrodon halys venom
InactiveCN111298072AGood curative effectNo adverse reactionAntipyreticAnalgesicsBidens pilosaAgkistrodon acutus
The invention discloses a traditional Chinese medicine composition for resisting agkistrodon halys venom. The traditional Chinese medicine composition is composed of the following raw materials: rhizoma paridis, herba lobeliae chinensis, herba bidentis bipinnatae, herba hedyotis, rhizoma ligustici Chuanxiong, radix angelicae dahuricae, radix et rhizoma rhei, turmeric, caulis spatholobi, and radixsaposhnikoviae. The traditional Chinese medicine composition provided by the invention is suitable for patients bitten by agkistrodon halys, has no adverse reactions, and has a rapid curative effect.
Owner:高云水 +1
A blood coagulation factor X activator and its preparation method
ActiveCN109207461BHigh purityExtended service lifePeptide/protein ingredientsBlood disorderElectrophoresesCOAGULATION FACTOR X
The invention provides a blood coagulation factor X activator, which is derived from bothrops atrox snake venom, and the invention also provides a preparation method of the blood coagulation factor X activator. The present invention also provides the application of the blood coagulation factor X activator in the preparation of medicine for treating bleeding disorders and a pharmaceutical composition for treating bleeding. Compared with the prior art, the blood coagulation factor X activator provided by the present invention has high purity, and the purity is 100% when detected by the size exclusion chromatography SEC column, and the purity is as high as 96% when detected by the C4 reverse-phase column. The method provided by the present invention first uses anion-cation exchange packing chromatography, and then performs affinity chromatography or composite packing chromatography, which not only can make the purity of coagulation factor X activator close to electrophoretic pure after affinity column chromatography, but also greatly extend the The service life of the affinity filler is extended, thereby saving the production cost and being conducive to large-scale industrial production.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Traditional Chinese medicine for preventing and treating cerebral arterial thrombosis
InactiveCN112263648ASafe to takeGood effectMammal material medical ingredientsCardiovascular disorderSalvia miltiorrhizaRadix Astragali seu Hedysari
The invention discloses a traditional Chinese medicine for preventing and treating cerebral arterial thrombosis. The traditional Chinese medicine comprises the following raw material components in mass ratio: 1000 of agkistrodon halys powder, 1000 of radix astragali powder, 500 of caulis spatholobi powder, 100 of hirudo powder, 100 of pheretima powder, 100 of rhizoma chuanxiong powder, 100 of radix salvia miltiorrhiza powder, 100 of rhizoma gastrodiae powder and 1 of moschus powder. Agkistrodon halys and radix astragali are monarch drugs and have the effects of tonifying qi and activating blood; rhizoma gastrodiae, hirudo and radix salviae miltiorrhizae are used as ministerial drugs for eliminating phlegm and removing blood stasis; rhizoma chuanxiong, caulis spatholobi, pheretima and moschus are assistant and guide each other to activate blood and promote blood circulation and enter brain orifices; and all the medicines are combined to achieve the effects of promoting blood circulation, removing blood stasis, tonifying deficiency and dredging collaterals, and the traditional Chinese medicine is safe to take and free of side effects, and can be used for preventing and treating cerebral arterial thrombosis.
Owner:大连正源中医门诊部有限公司
Mutant Alfimeprase engineering strain, and preparation method and applications thereof
ActiveCN102080099BSame thrombolytic activityN-terminal stabilizationEnzymesFermentationEnzyme GeneNucleotide
The invention relates to a preparation method of a recombinant viper venom protein. A mutant Alfimeprase gene of artificially synthesized Agkistrodon contortrix is utilized, and the gene has the nucleotide sequence shown as SEQ ID No.2. The gene is linked to yeast expression plasmids pPIC9K to construct a high efficiency expression vector pPIC9K-His-Alf, a Pichia yeast strain GS115 is introduced,and the vector is named as FY-Alf. Induced expression is carried out with methanol, the expressed recombinant protein is purified and then subjected to activity verification by a fibrin plate assay, and the result shows that the recombinant protein Alfimeprase expressed by the yeast strain GS115 containing pPIC9K-His-Alf plasmids has high fibrinolytic activity.
Owner:ANHUI FENGYUAN PHARM CO LTD
A haemocoagulase sublingual film of Agkistrodon lancehead and preparation method thereof
ActiveCN109331175BAvoid destructionAvoid first pass effectPeptide/protein ingredientsMacromolecular non-active ingredientsOral medicationPharmaceutical drug
Owner:AIR FORCE MEDICAL CENT PLA
Method for preparing venom edema factor inhibition protein and application thereof
InactiveCN101607991BFree from harmEasy to purifyAntinoxious agentsMicroorganism based processesDiseaseGloydius shedaoensis
The invention relates to method for preparing venom edema factor inhibition protein and the application thereof, belonging to the field of biotechnology and relating to a method for separating and purifying a protein having inhibition action on venom edema factors from the blood serum of Gloydius shedaoensis. The invention is characterized by the method adopted for separating and purifying the inhibition protein, and the method comprises the following steps: performing the ammonium sulphate precipitation on the crude blood serum of Gloydius shedaoensis, performing gel filtration twice, passing through an anion exchange column, an affinity chromatography column and a reversed phase HPLC to obtain a single component with the molecular weight of 25KDa, and identifying 70 amino acid sequencesof the N terminal and 20 amino acid sequences of the C terminal of the protein. The invention is also characterized by obtaining the gene sequence corresponding to the venom edema factor inhibition protein, the gene sequence is established on a TOPO expression vector for producing in vitro and expressing the protein. The invention has the advantages of providing a preparation method for producingin vitro venom edema factor inhibition protein, and the inhibition protein can be applied to the clinical treatment of snakebite and other diseases.
Owner:DALIAN UNIV OF TECH
Reversed-phase HPLC (High Performance Liquid Chromatography) fingerprint detection method for snake venom of Changbai Mountain
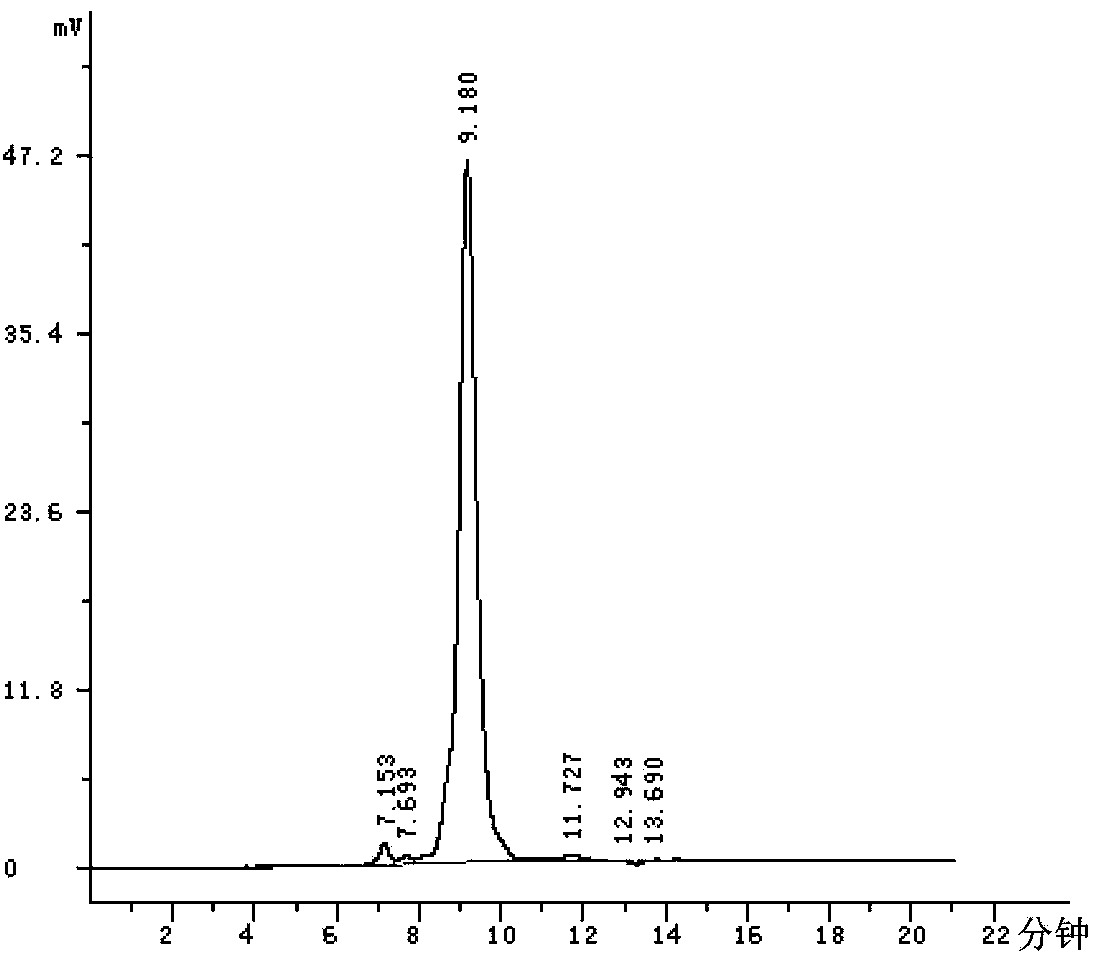
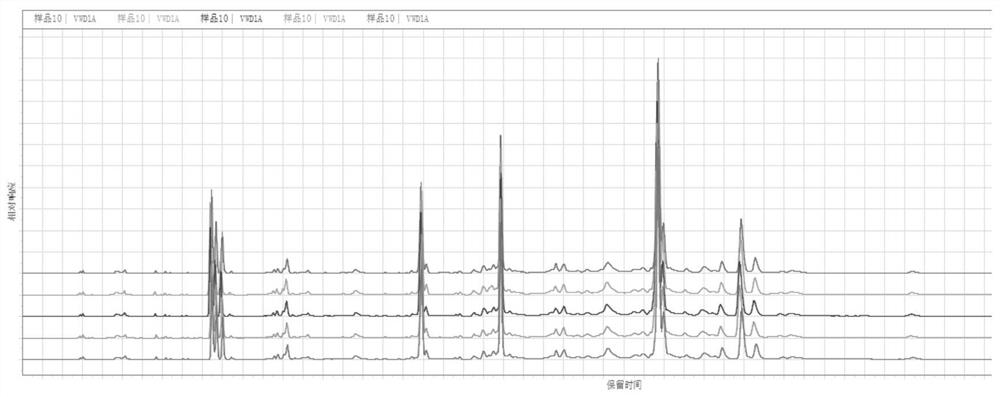
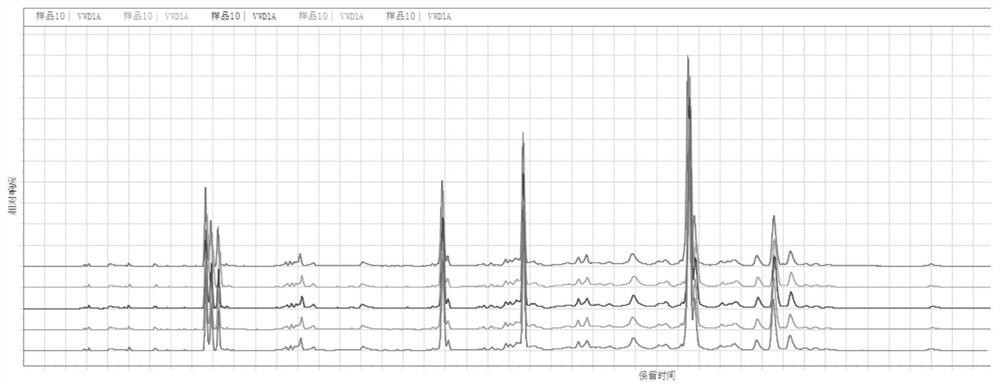
PendingCN114689703AEasy to operateGood reproducibilityComponent separationTest sampleFingerprint detection
The invention belongs to the technical field of medicines, and particularly relates to a Changbai Mountain white agkistrodon halys venom fingerprint detection method, which comprises the following steps: (1) preparation of a test solution and a reference solution: respectively taking a Changbai Mountain white agkistrodon halys venom test solution and a Changbai Mountain white agkistrodon halys venom reference solution, dissolving with a solvent, diluting, centrifuging, taking supernate, and filtering; and (2) fingerprint detection: respectively injecting the test sample and the reference substance solution obtained in the step (1) into a liquid chromatograph, recording chromatograms, and calculating the similarity by adopting a traditional Chinese medicine chromatographic fingerprint similarity evaluation system. The detection method for the snake venom fingerprint spectrum of the Changbai Mountain white agkistrodon halys has the advantages of strong operability, good reproducibility and high specificity, can be used as a standard method for detecting and identifying the snake venom of the Changbai Mountain white agkistrodon halys, and can provide more comprehensive quality information of the snake venom of the Changbai Mountain white agkistrodon halys.
Owner:JINZHOU AHON PHARM CO LTD
Marker peptide of snake venom thrombin-like enzymes (svtles) from agkistrodon halys pallas and application thereof
ActiveUS20220034856A1The process is convenient and fastQuantitative precisionComponent separationEnzymesLiquid chromatography mass spectroscopyMass analyzer
The present invention relates to the field of chemical analysis detection and application, in particular to a marker peptide of snake venom thrombin-like enzymes (SVTLEs) from Agkistrodon Halys Pallas and an application thereof. The amino acid sequence of the marker peptide of snake venom thrombin-like enzymes (SVTLEs) from Agkistrodon Halys Pallas is TLCAGVMEGGIDTCNR. Characterizing the source of species and a content of the SVTLEs in a to-be-detected sample by using the marker peptide includes the following steps of: pretreating the to-be-detected sample by trypsin through enzymolysis, and taking a supernatant of an enzymolysis liquid as a test solution; and injecting the test solution and a reference solution into a liquid chromatography-mass spectrometer, and selecting a qualitative ion pair and a quantitative ion pair for detecting the source of species and a content of the SVTLEs in the to-be-detected sample.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com