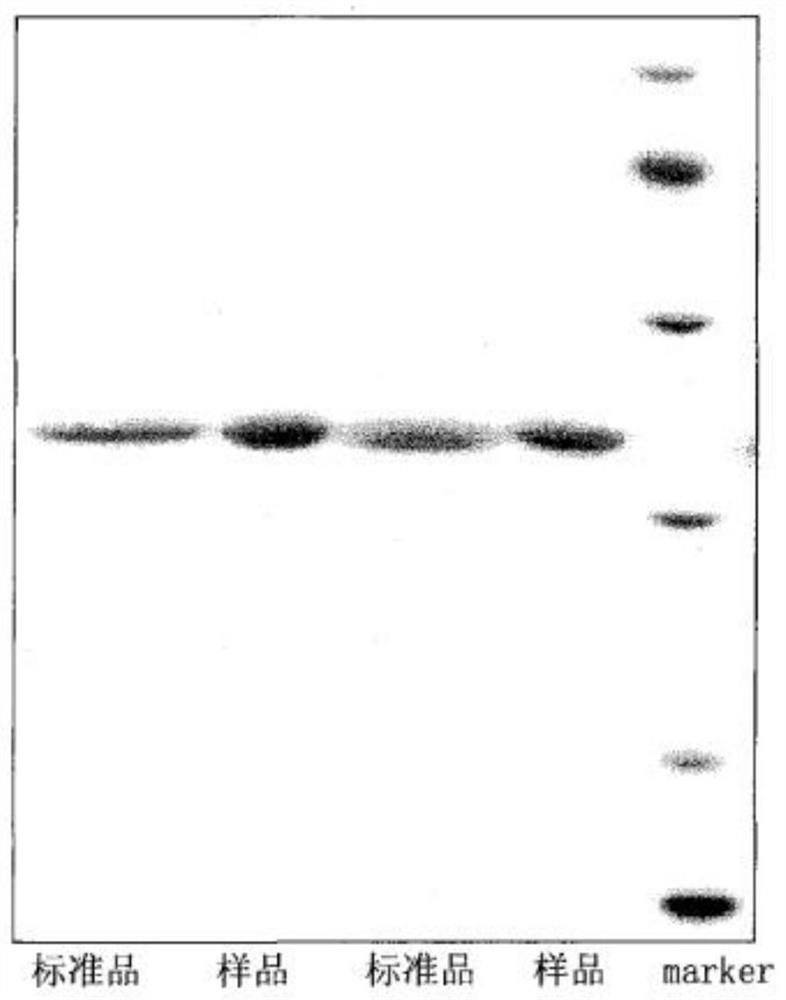
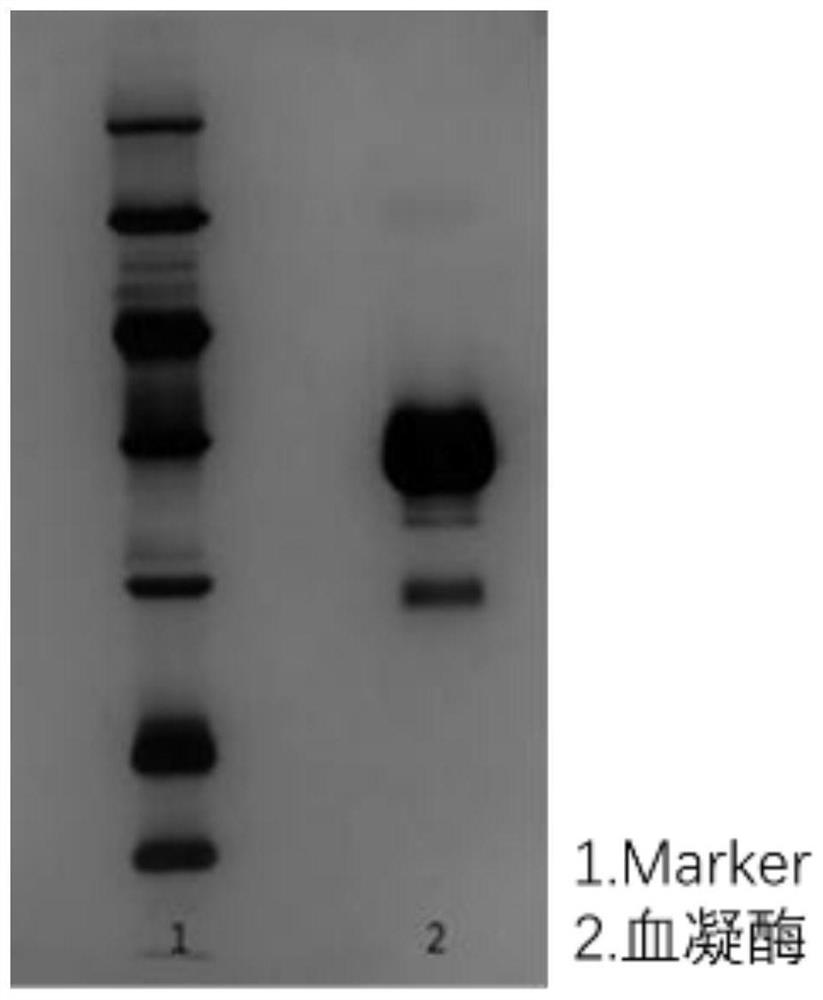
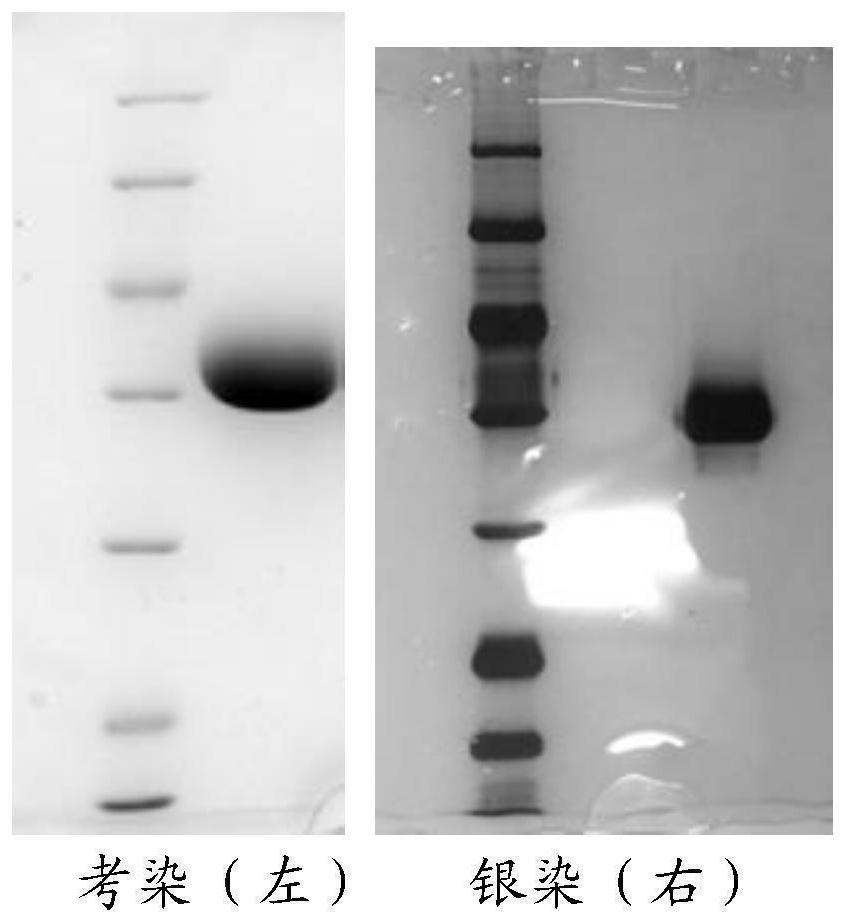
Method for purifying hemagglutinin of spearhead agkistrodon halys
A purification method and hemocoagulase technology, applied in the field of hemocoagulase purification, can solve the problems of low specific activity of pure hemocoagulase, deviation of hemocoagulase purity, inconvenient large-scale production, etc., and achieve consistent product quality Good sex, avoid immunogenic reaction, low protein effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Purification of hemagglutinin using the method of the present invention
[0071] 1. Dissolve 5g of the venom of the spearhead pit viper in 50mL of 20mM Tris-HCl, 0.2M NaCl, pH8.0 buffer, soak it at room temperature to fully dissolve it, and then centrifuge at 5000rpm for 5min to remove the precipitate and recover the supernatant .
[0072] 2. Benzamidine Sepharose 6B affinity chromatography column (2.6cm×50cm) was equilibrated for 3 column volumes with 20mM Tris-HCl, 0.2M NaCl, pH8.0 buffer, and then centrifuged The supernatant of the snake venom was loaded onto the chromatography column; after the loading, the buffer was continuously washed for 3 column volumes, and eluted with an eluent of 20 mM Tris-HCl, 0.2 M NaCl, 1 M arginine, pH 8.0 , collect the target components.
[0073] 3. The target component after affinity chromatography was ultrafiltered and exchanged into a buffer of 20 mM PB, pH 6.0, and the molecular weight cut-off of the ultrafiltration mem...
Embodiment 2
[0078] Example 2 Purification of hemagglutinin using the method of the present invention
[0079] 1. Dissolve 5 g of the venom from the venom of the spearhead pit viper in 30 mL of 50 mM Tris-HCl, 0.1 M NaCl, pH 8.0 buffer, soak it at room temperature to fully dissolve it, and then centrifuge at 5000 rpm for 5 min to remove the precipitate and recover the supernatant. .
[0080] 2. Benzamidine Sepharose 6B affinity chromatography column (2.6cm×50cm) was equilibrated for 3 column volumes with 50mM Tris-HCl, 0.1M NaCl, pH7.0 buffer, and then centrifuged The supernatant of the snake venom was loaded onto the chromatography column; after the loading, continued to wash with buffer for 3 column volumes, and eluted with the eluent of 50mM Tris-HCl, 0.1M NaCl, 0.2M arginine, pH 6.0 , collect the target components.
[0081] 3. The target component after affinity chromatography was ultrafiltered and exchanged into a buffer of 5mM PB, pH 6.2, and the molecular weight cut-off of the ult...
Embodiment 3
[0086] Example 3 Purification of hemagglutinin using the method of the present invention
[0087] 1. Dissolve 5 g of the venom of the spearhead pit viper in 60 mL of 100 mM Tris-HCl, 0.5 M NaCl, pH 9.0 buffer, soak it at room temperature to fully dissolve it, and then centrifuge at 5000 rpm for 5 min to remove the precipitate and recover the supernatant. .
[0088] 2. Benzamidine Sepharose 4B affinity chromatography column (2.6cm×50cm) was equilibrated for 3 column volumes with 100mM Tris-HCl, 0.5M NaCl, pH9.0 buffer, and then centrifuged The supernatant of snake venom was loaded onto the chromatography column; after the loading, continued to wash with buffer for 3 column volumes, and eluted with the eluent of 100mM Tris-HCl, 0.5M NaCl, 0.05M arginine, pH 9.0 , collect the target components.
[0089] 3. The target component after affinity chromatography was ultrafiltered and exchanged into a buffer of 50 mM PB, pH 6.1, and the molecular weight cut-off of the ultrafiltration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com