Pharmacokinetics-pharmacodynamics analysis method for treating chronic heart failure by using astragalus membranaceus and ginseng granules based on metabonomics
A chronic heart failure and pharmacokinetic technology, applied in the field of analysis, can solve the problems of insufficient index components and efficacy indicators, complex chemical components of traditional Chinese medicine compounds, and insufficient correlation between index components and efficacy indicators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Materials and Instruments
[0058] 1.1 Drugs, reagents and instruments
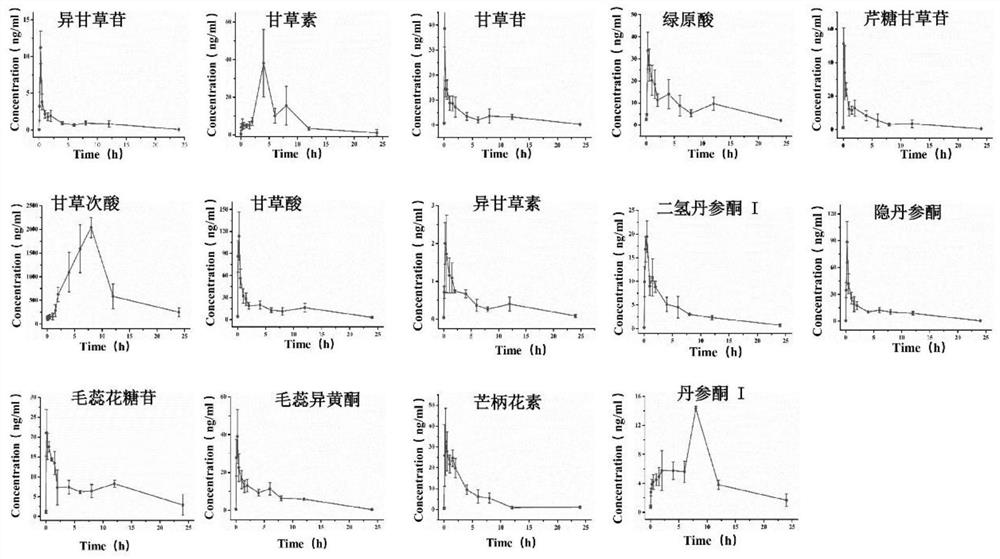
[0059] Tanshinone I (purity>98%, batch number: 170926), dihydrotanshinone I (purity>98%, batch number: 180109), cryptotanshinone (purity>98%, batch number: 171029), formononetin (purity>98% , batch number: 170328), actecoside (purity>98%, batch number: 170730), chlorogenic acid (purity>98%, batch number: 171120), verbascoside (purity>98%, batch number: 170920), glycyrrhizic acid (purity >98%, batch number: 181226), glycyrrhetinic acid (purity>98%, batch number: 170910), liquiritigenin (purity>98%, batch number: 181227), isoliquiritigenin (purity>98%, batch number: 190226), Liquiritin (purity>98%, batch number: 170330), isoliquiritin (purity>98%, batch number: 190317), apiose liquiritin (purity>98%, batch number: 190107) were purchased from Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd. Company; Standards Naringin and Reserpine and Endogenous Standards L-Tryptophan, Xanthine Ac...
Embodiment 2
[0080] Embodiment 2 pharmacokinetic research results and discussion
[0081] 1. Methodology
[0082] 1. Optimization of mass spectrometry conditions
[0083] The prepared mixed standard solution was diluted to a concentration of 1 μg / mL for optimization of mass spectrometry conditions. The MRM parameters of each analyte are shown in Table 1.
[0084] 2. Linearity of the method
[0085] Use concentration of 0.01, 0.025, 0.05, 0.1, 1, 5, 10, 50, 100, 250, 500, 1000, 2500, 5000ng·mL -1 standard solution to draw a standard curve. Taking the peak area ratio (Y) of the analyte and the internal standard in the plasma sample as the ordinate, and the concentration (X) of the analyte as the abscissa, the least squares regression method is used to prepare the calibration curve, and the regression of each compound is obtained. Equation, correlation coefficient (R 2 ) and linear range. The lower limit of quantification (LLOQ) was calculated with the signal-to-noise ratio S / N=3, and S / ...
Embodiment 3
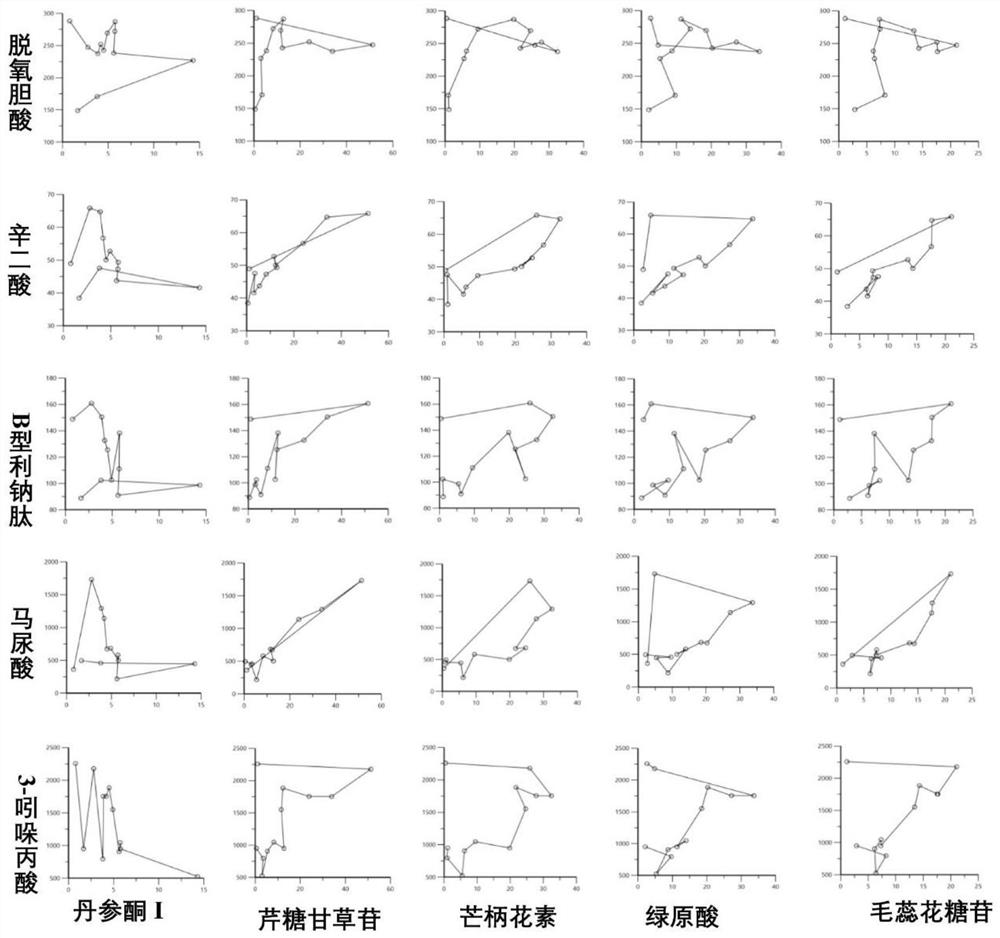
[0107] Example 3 Establishment of PK-PD binding model
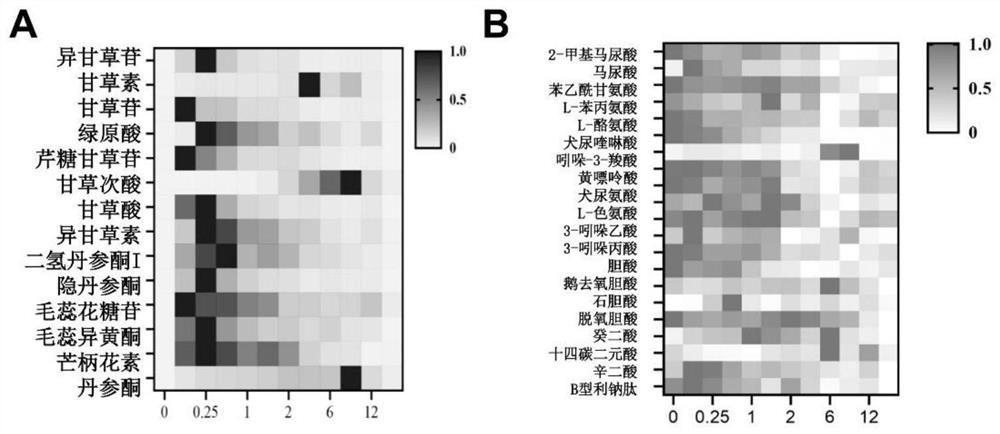
[0108] The association analysis results of the 5 active components of Qishen Granules in plasma and the 4 endogenous markers in the metabolic pathways related to CHF and BNP by Phoenix WinNonlin 6.0 software are as follows: image 3 shown. Each endogenous substance can show a clockwise or counterclockwise change with the concentration of the four active ingredients, indicating that the relationship between the active ingredients and the endogenous indicators is not a simple linear relationship, showing complex interrelationships and PK indicators The delayed effect of Qishen Granules indicates that the active ingredients of Qishen Granules play a combined and synergistic effect on the treatment of CHF, which provides a new perspective for elucidating the active substances of Qishen Granules and their mechanism of treating CHF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com