Metallocene catalyst and preparation method and application thereof
A technology of metallocene catalyst and co-catalyst, which can be used in the fields of metallocene catalyst preparation of polyolefin, metallocene catalyst preparation, and metallocene catalyst field, and can solve the problems of low copolymerization ability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
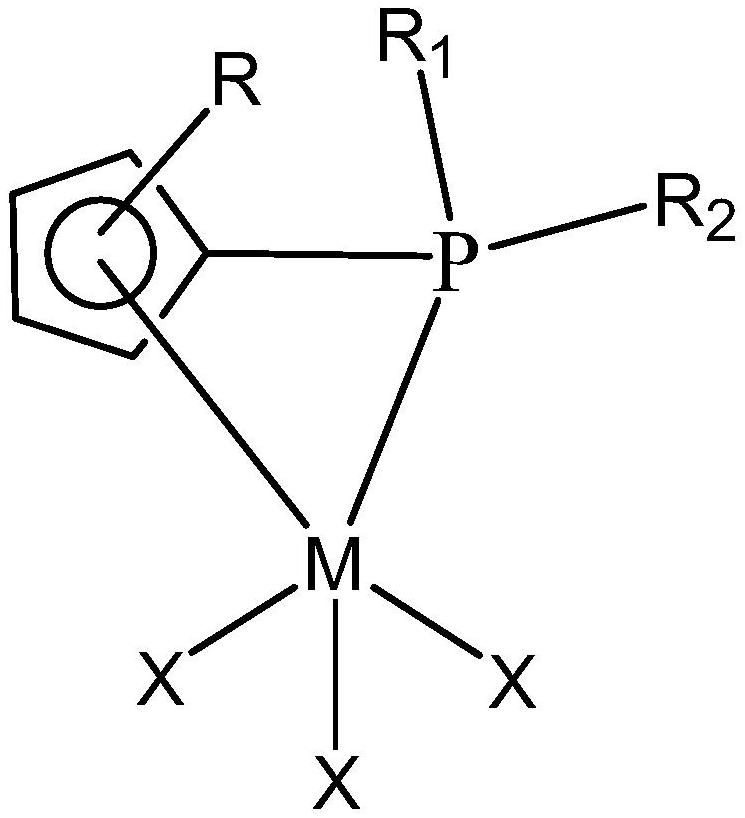
[0046] (1) Synthesis of ligand compound L1 [L1 structure: R in general formula (6) takes H, R 1 , R 2 Take phenyl]
[0047] Take 40ml of tetrahydrofuran and 10ml of cyclopentadiene in a 300ml schlenk bottle, add 0.69g of sodium metal at -10°C, stir, and react for 8 hours, add diphenylphosphine chloride equal to the mole of sodium metal, and -30°C After reacting for 6 hours, the solvent was removed in vacuo, 100ml of n-hexane was added to the remaining solid, dissolved and filtered, and the filtrate was recrystallized to obtain 4.87g of cyclopentadienyl diphenylphosphine solid, yield: 64.9%. 1 H NMR (400MHz, CDCl 3 :7.26ppm): δ=7.42(m,4H,Ph); 7.33(m,6H,Ph); 6.99(d,2H,Cp); 6.65(m,2H,Cp); 3.48(t,1H,Cp ); Anal.Calcd.(%) for C 17 h 15 P(250): C, 81.58; H, 6.04; found: C, 81.56; H, 6.07; ESI-MS m / z calculated for [M+H] + .C 17 h 15 P: 250.09, found, 251.09.
[0048] (2) Preparation of main catalyst
[0049] Take 20ml of toluene and 1g of ligand L1 in a 300ml schlenk bottle...
Embodiment 2
[0055] (1) Synthesis of ligand compound L2 [L2 structure: in the general formula (6) Take tetramethylcyclopentadiene, R 1 , R 2 isopropyl]
[0056] Take 40ml of tetrahydrofuran and 3.66g of tetramethylcyclopentadiene in a 300ml schlenk bottle, add 0.69g of sodium metal at -10°C, stir, react for 8 hours, add diisopropylphosphine chloride equal to the mole of sodium metal , react with -30°C for 6 hours, remove the solvent in vacuum, add 100ml of n-hexane to the remaining solid, dissolve and filter, and recrystallize the filtrate to obtain 4.97g of tetramethylcyclopentadienyl diisopropylphosphine solid, the yield : 69.5%. 1 H NMR (400MHz, CDCl 3 :7.26ppm): δ=3.56(m, 1H, Cp); 1.98(s, 6H, Cp-CH 3 ); 1.92(m,2H,-CH); 1.85(d,6H,Cp-CH 3 ); 1.05(d,12H,P-CH 3 ); Anal.Calcd.(%) for C 15 h 27 P(238): C,75.59; H,11.42; found: C,75.61; H,11.41; ESI-MS m / z calculated for [M+H] + .C 15 h 27 P: 238.19, found, 239.19.
[0057] (2) Preparation of main catalyst
[0058] Take 20ml of...
Embodiment 3
[0064] (1) Synthesis of ligand compound L3 [L3 structure: R in general formula (6) takes H, R 1 , R 2 Methyl]
[0065] Take 40ml of tetrahydrofuran and 10ml of cyclopentadiene in a 300ml schlenk bottle, add 0.69g of sodium metal at -10°C, stir, and react for 8 hours, add dimethylphosphine chloride equal to the molarity of sodium metal, and -30°C After reacting for 6 hours, the solvent was removed in vacuo, 100ml of n-hexane was added to the remaining solid, dissolved and filtered, and the filtrate was recrystallized to obtain 2.89g of cyclopentadienyl dimethylphosphine solid, yield: 76.4%. 1 H NMR (400MHz, CDCl 3 :7.26ppm): δ=6.65(d,2H,Cp); 6.22(m,2H,Cp); 3.68(m,1H,Cp); 0.98(d,6H,P-CH 3 ); Anal.Calcd.(%) for C 7 h 11 P(126): C,66.65; H,8.79; found: C,66.63; H,8.80; ESI-MS m / z calculated for [M+H] + .C 7 h 11 P: 126.06, found, 127.05.
[0066] (2) Preparation of main catalyst
[0067] Take 20ml of toluene and 1g of ligand L3 in a 300ml schlenk bottle, add n-butyllithi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com