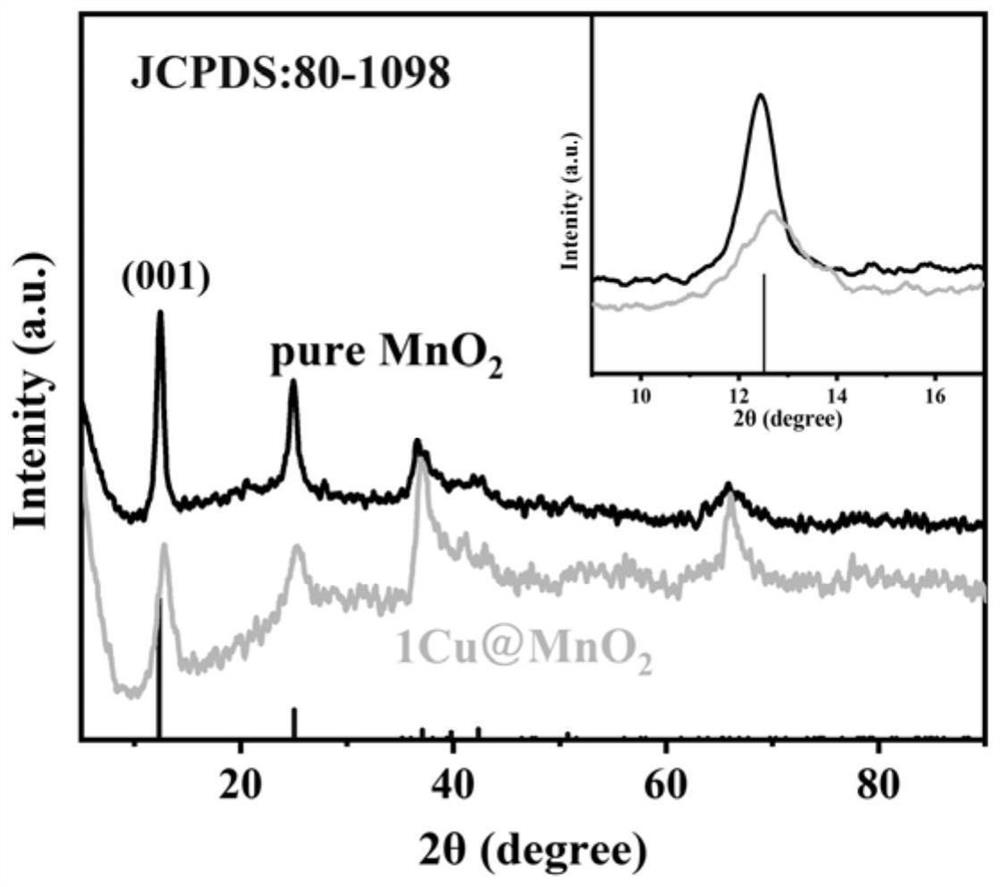
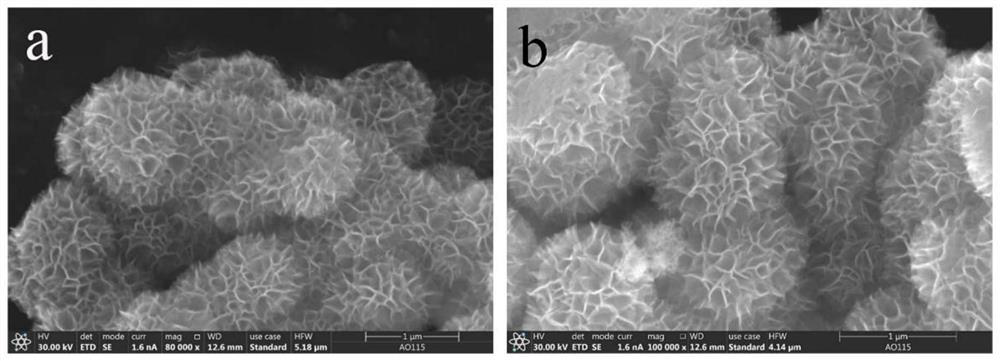
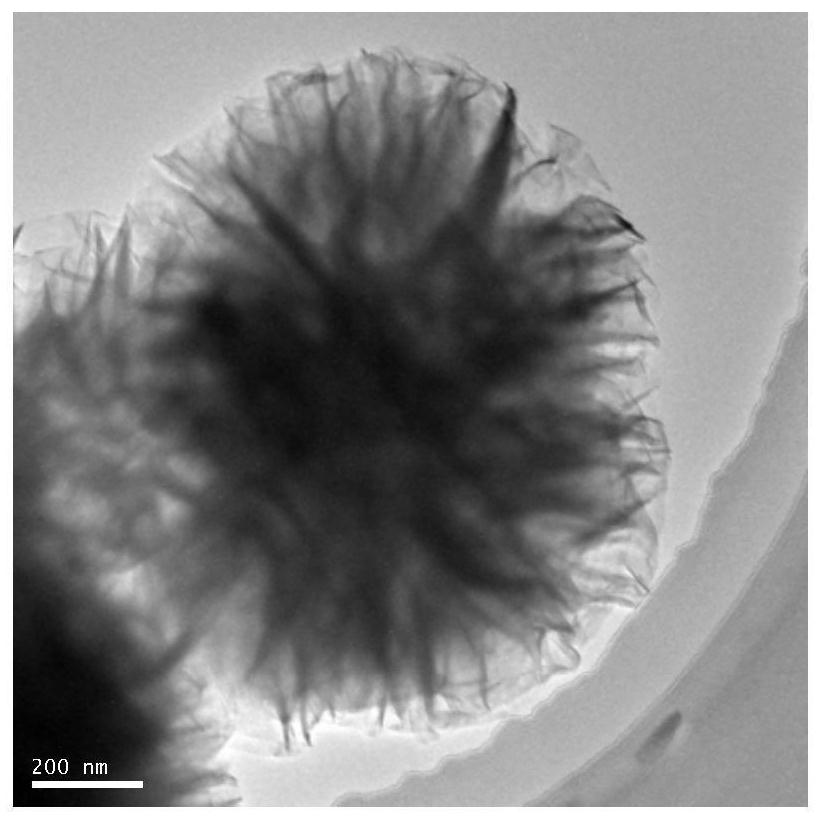
A kind of preparation method and application of high stability copper intercalation manganese dioxide electrode material
A manganese dioxide electrode, high-stability technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of poor cycle stability of layered manganese dioxide electrode materials, and cannot improve cycle stability and rate performance at the same time. and other problems, to achieve the effect of improving electrochemical performance, excellent electrochemical performance, and improving electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of a high-stability copper intercalated manganese dioxide electrode material of the present embodiment is carried out according to the following steps:
[0047] Dissolve 1mmol, 0.1691g of manganese sulfate in 40mL of deionized water, stir magnetically for 20 minutes, then dissolve 0.1mmol, 0.0250g of copper source in the manganese sulfate solution, and stir to form a first mixed solution; then 6mmol , 0.9482g of potassium permanganate was dissolved in 40mL of deionized water, magnetically stirred for 20 minutes, the potassium permanganate solution was slowly dropped into the first mixed solution, and the second mixed solution was formed after magnetic stirring for 1 h, and then the first mixed solution was added. The two mixed solutions were moved into the lining of the autoclave, put into the autoclave, put into an oven, and reacted at 160° C. for 12 hours. After the reaction is completed, take out and centrifuge with deionized water and vacuum d...
Embodiment 2
[0051] The preparation method of a high-stability copper intercalated manganese dioxide electrode material of the present embodiment is carried out according to the following steps:
[0052] Dissolve 1mmol, 0.1691g of manganese sulfate in 40mL of deionized water, stir magnetically for 20 minutes, then dissolve 0.5mmol, 0.125g of copper source in the manganese sulfate solution, and stir to form a first mixed solution; then 6mmol , 0.9482g of potassium permanganate was dissolved in 40mL of deionized water, magnetically stirred for 20 minutes, the potassium permanganate solution was slowly dropped into the first mixed solution, and the second mixed solution was formed after magnetic stirring for 1 h, and then the first mixed solution was added. The two mixed solutions were moved into the lining of the autoclave, put into the autoclave, put into an oven, and reacted at 160° C. for 12 hours. After the reaction was completed, the precipitate was obtained by centrifugation with deion...
Embodiment 3
[0056] The preparation method of a high-stability copper intercalated manganese dioxide electrode material of the present embodiment is carried out according to the following steps:
[0057] Dissolve 1mmol, 0.1691g of manganese sulfate in 40mL of deionized water, stir magnetically for 20 minutes, then dissolve 1mmol, 0.2497g of copper source in the manganese sulfate solution, and stir to form a first mixed solution; then 6mmol, 0.9482 g of potassium permanganate was dissolved in 40 mL of deionized water, magnetically stirred for 20 minutes, the potassium permanganate solution was slowly dropped into the first mixed solution, and the second mixed solution was formed after magnetic stirring for 1 h. The mixed solution was moved into the lining of the autoclave, put into the autoclave and put into an oven, and reacted at 160° C. for 12 hours. After the reaction was completed, the precipitate was obtained by centrifugation with deionized water, and vacuum-dried at 80 °C for 12 h t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com