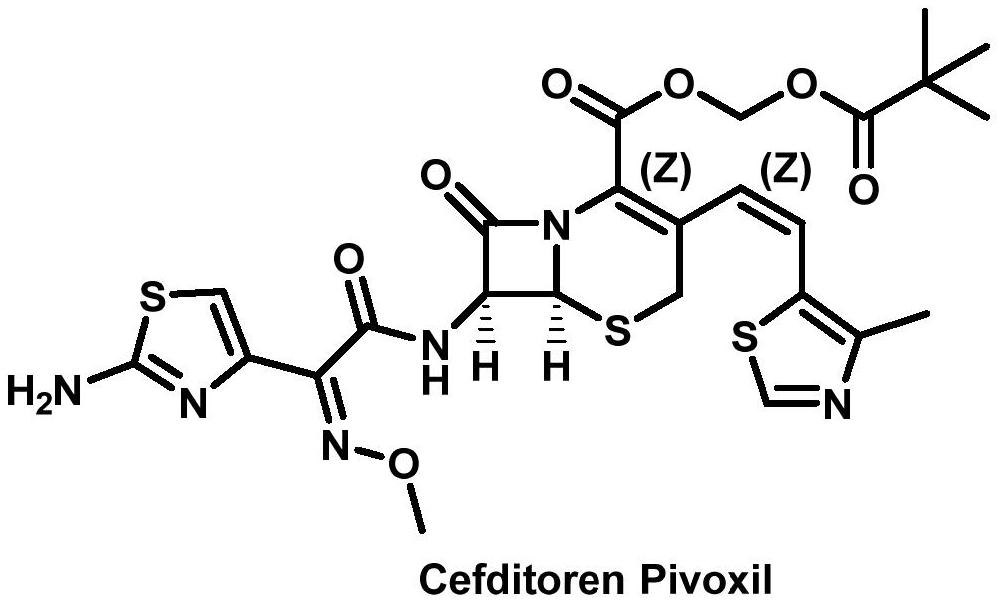
Preparation method of cefditoren pivoxil ring-opening dimer
A technology of cefditoren pivoxil and cefditoren pivoxil, which is applied in the field of preparation of cefditoren pivoxil ring-opening dimer, can solve the problems of limited popularization and application, harsh reaction conditions, long reaction route, etc., and achieve raw material economy Easy to obtain, simple reaction and operation, and the effect of reducing synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
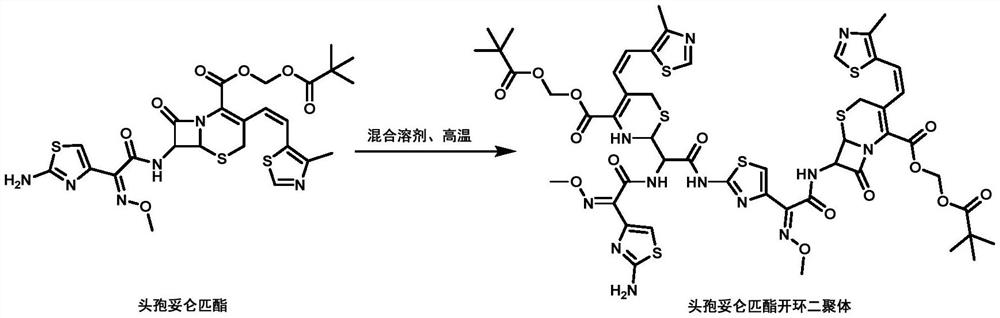
[0027] This embodiment provides a kind of preparation method of cefditoren pivoxil ring-opening dimer, the specific steps are as follows:
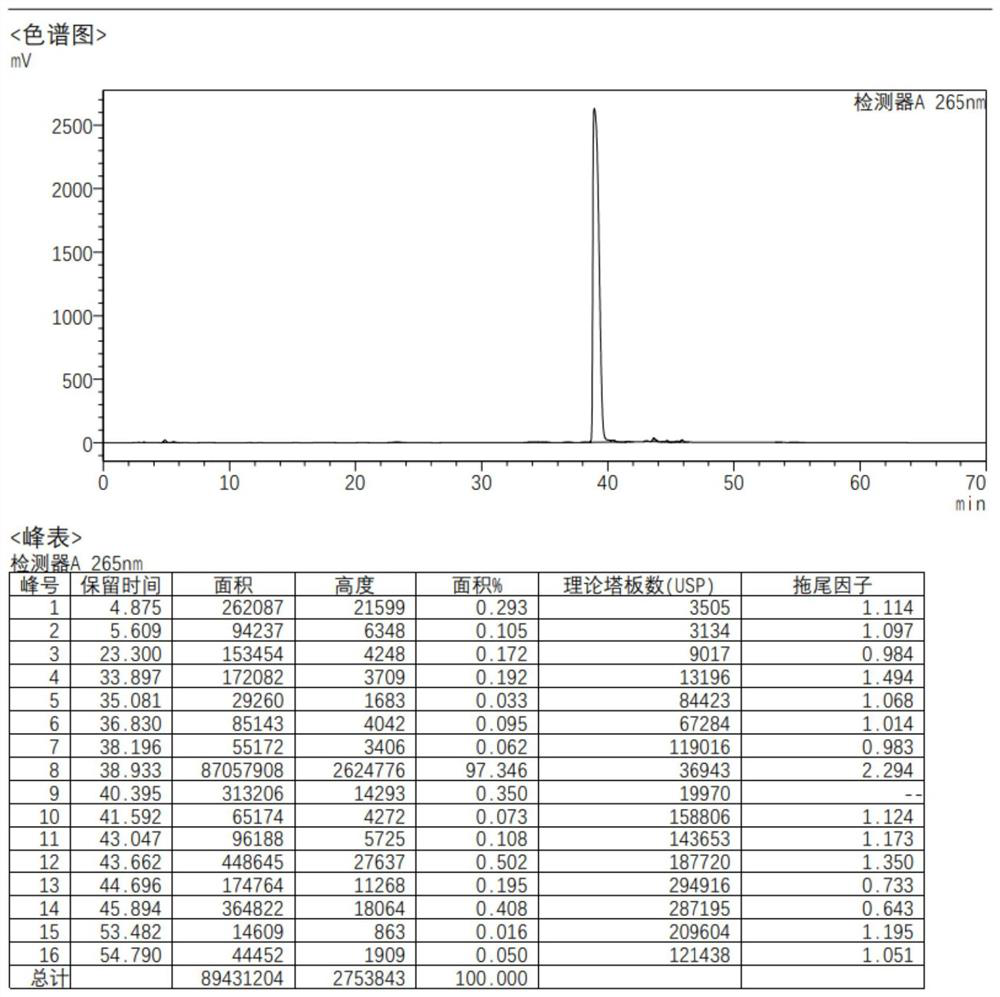
[0028] At room temperature, a mixed solvent with a dichloromethane / ethanol volume ratio of 5:1 was prepared. Get cefditoren pivoxil 20g (32.2mmol), join in 200mL mixed solvent, stir until dissolving. The temperature was raised to 55° C. for the reaction, and the peak of the ring-opened dimer of cefditoren pivoxil was monitored by HPLC, and the reaction was terminated. After cooling down to room temperature, the solvent was evaporated to dryness using a rotary concentrator to obtain a light yellow crude product. Purified by silica gel column chromatography, eluted with dichloromethane-methanol 80:1 to obtain a light yellow solid, transferred to an oven at 40°C and dried under reduced pressure for 3 to 5 hours to obtain 8.5 g of dry product, the purity of which was detected by HPLC was 97.3% (see figure 1 ).
Embodiment 2
[0030] This embodiment provides a kind of preparation method of cefditoren pivoxil ring-opening dimer, the specific steps are as follows:
[0031] At room temperature, a mixed solvent with a dichloromethane / methanol volume ratio of 8:1 was prepared. Get cefditoren pivoxil 20g (32.2mmol), join in 220mL mixed solvent, stir until dissolving. The temperature was raised to 50° C. for the reaction, and the peak of the ring-opened dimer of cefditoren pivoxil was monitored by HPLC, and the reaction was terminated. After cooling down to room temperature, the solvent was evaporated to dryness using a rotary concentrator to obtain a light yellow crude product. Purified by silica gel column chromatography, eluted with chloroform-methanol 70:1 to obtain a light yellow solid, transferred to an oven at 40°C and dried under reduced pressure for 3-5 hours to obtain 8.1 g of dry product, the purity of which was detected by HPLC was 95.4%.
Embodiment 3
[0033] This embodiment provides a kind of preparation method of cefditoren pivoxil ring-opening dimer, the specific steps are as follows:
[0034] At room temperature, a mixed solvent with a volume ratio of chloroform / ethyl acetate of 10:1 was prepared. Get cefditoren pivoxil 20g (32.2mmol), join in 250mL mixed solvent, stir until dissolving. The temperature was raised to 65° C. for the reaction, and the peak of the ring-opened dimer of cefditoren pivoxil was monitored by HPLC, and the reaction was terminated. After cooling down to room temperature, the solvent was evaporated to dryness using a rotary concentrator to obtain a light yellow crude product. Purified by silica gel column chromatography, eluted with dichloromethane-ethanol 100:1 to obtain a light yellow solid, transferred to an oven at 40°C and dried under reduced pressure for 3-5 hours to obtain 8.3 g of a dry product, the purity of which was detected by HPLC was 96.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com