Freeze-dried powder and preparation method and application thereof
A technology of freeze-drying and powder, which is applied in the direction of pharmaceutical formulations, preparations for in vivo tests, echo/ultrasonic imaging agents, etc., can solve the problems of limited production batches, difficulties in large-scale production, and high energy consumption in production, and achieve the number of microbubbles Ideal particle size, increased production volume, and reduced production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] This experimental example provides a method for preparing freeze-dried microbubbles in the prior art, and compares the effects of the two freeze-drying methods on the effect of the product.
[0032] 1. Preparation of lyophilized microbubbles:
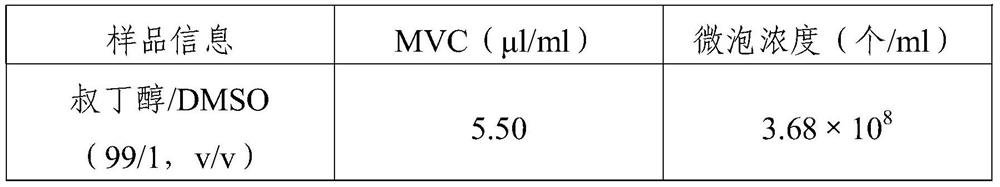
[0033] Prepare freeze-dried microbubbles according to the method disclosed in Example 2 of Patent CN112165959 A: add 190mg DSPC, 190mg DPPG-Na and 40mg PA to 84ml hexane / ethanol (8 / 2, v / v) mixed solvent, stir until dissolved, Evaporate the solvent to dryness under vacuum, add 24.56g PEG4000, add 500ml tert-butanol after mixing, heat up to about 60°C to dissolve, filter with a 0.22μm filter membrane, fill it into an 8ml injection bottle, half-press the stopper, and use quick-freezing (pre- Freezing temperature -45°C) and slow freezing (pre-freezing temperature -30°C) for freeze-drying, the specific freeze-drying procedures are: 1) Pre-freezing: the shelf is pre-cooled to -45°C or -30°C and placed in The sample was maintained for ...
Embodiment 1
[0041] This example provides a preparation method of the freeze-dried powder of the present invention, and its effect is verified.
[0042] 1. Preparation and Evaluation of Lyophilized Microbubbles
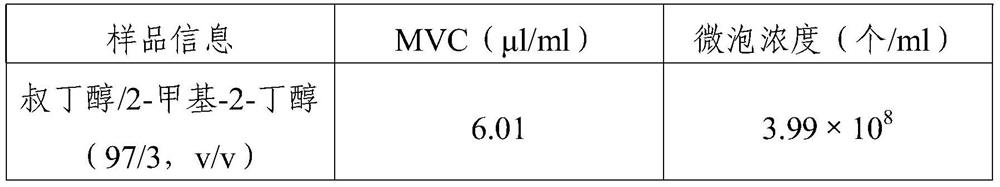
[0043]The preparation of layered phospholipids and the steps of mixing with PEG4000 are the same as those in Experimental Example 1. After that, add 500ml of tert-butanol / 2-methyl-2-butanol (97 / 3, v / v) mixed solvent, heat up to about 60°C to dissolve, filter with a 0.22μm membrane, and fill it into an 8ml injection bottle. The freeze-dried microbubbles were prepared by the pre-freezing method of slow freezing (-30°C) (the specific freeze-drying procedure was the same as that of Experimental Example 1). 6 Saturate and seal the injection vial with a rubber stopper. The evaluation method of the aerated microbubble suspension is the same as that of Experimental Example 1.
[0044] 2. Results analysis, see Table 2:
[0045] Table 2
[0046]
Embodiment 2
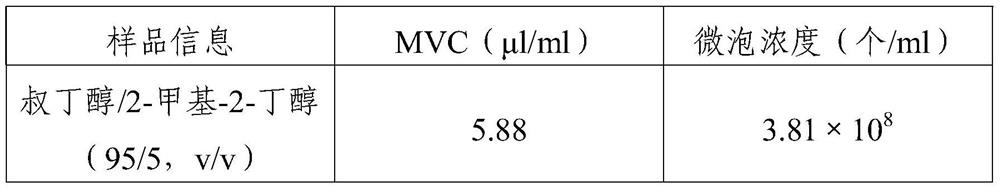
[0048] This example provides a preparation method of the freeze-dried powder of the present invention, and its effect is verified. The specific preparation and evaluation method of freeze-dried microbubbles are the same as those in Example 1, except that the freeze-drying solvent is selected from tert-butanol and 2-methyl-2-butanol, the volume ratio of the two is 95:5.
[0049] The evaluation results are shown in Table 3:
[0050] table 3
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com