Dihydroporphin compound as well as preparation method and application thereof
A compound and hydrate technology, applied in the field of medicine, can solve the problems of uncontrollable quality, different physiological activities, unclear configuration, etc., and achieve the effects of less toxic and side effects, good safety and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
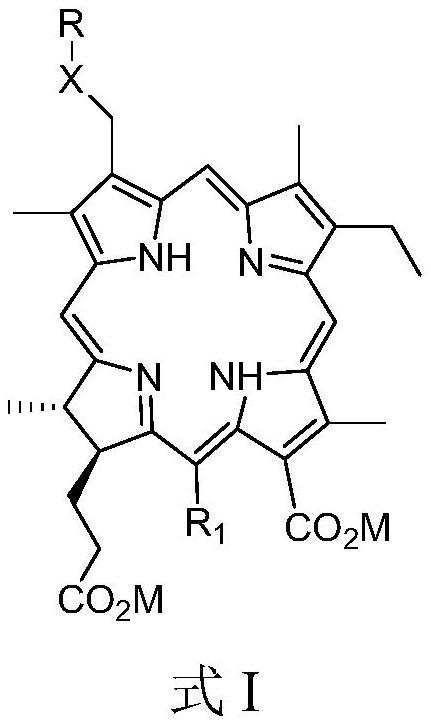
[0106] The preparation of embodiment 1,3-hexyloxymethyl-3-devinyl chlorin e4
[0107] (1) Synthesis of chlorin e4 dimethyl ester
[0108] Weigh 10.0g (18.12mmol) of chlorin e4 (CAS No.550-52-7), dissolve in 200mL of methanol and toluene (1:1 volume ratio) mixed solution, slowly drop (3eq ) 27.3mL trimethylsilyldiazomethane in n-hexane solution (concentration is 2mol / L), stirred at room temperature for 3h. TLC check, after the reaction is complete, add 0.5mL glacial acetic acid and stir for 10min to quench the reaction. The solvent was distilled off under reduced pressure and extracted with ethyl acetate and water (1:1, v / v). The ethyl acetate layer was concentrated under reduced pressure to obtain a crude product. The crude product was subjected to normal-phase silica gel column chromatography and gradient elution with petroleum ether-ethyl acetate (volume ratio: 50:1 to 1:1), and a total of 9.5 g of dark green chlorin e4 dimethyl ester was obtained. The yield was 90.3%. H...
Embodiment 2
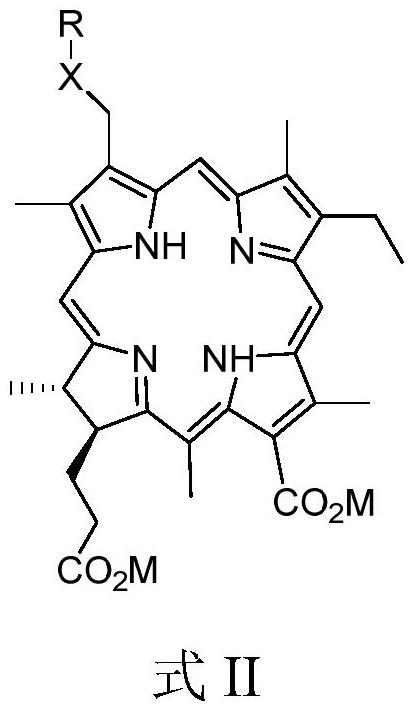
[0129] Embodiment 2, Preparation of 3-hexyloxymethyl-3-desvinyl chlorin e4 disodium salt
[0130] Weigh 160 mg (0.25 mmol) of 3-hexyloxymethyl-3-desvinyl chlorin e4, dissolve it in 100 mL of acetone-methanol (20:1 by volume) mixed solution, and then slowly add A slight excess of 1mol / L sodium hydroxide-methanol solution, after the dark green solid is completely precipitated, it is filtered to obtain 3-hexyloxymethyl-3-desvinyl chlorin e4 disodium salt (CEFO), the purity (HPLC): 99.86%. The compound structure is as follows:
[0131]
Embodiment 3-4
[0132] Embodiment 3-4: adopt the same method as embodiment 2, wherein respectively use potassium hydroxide and ammonia water instead of sodium hydroxide to prepare corresponding potassium salt and ammonium salt, other reaction conditions are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com