Preparation method of copper-iron bimetal confinement nitrogen-doped carbon nanotube composite material
A copper-iron bimetallic, nitrogen-doped carbon technology is applied in chemical instruments and methods, water/sludge/sewage treatment, water pollutants, etc. Uneven distribution of points, inability to scale production, etc., to achieve the effect of easy control of reaction process conditions, low catalytic activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Precursor preparation process: Take a certain amount of concentrated hydrochloric acid to prepare 20mL hydrochloric acid solution (concentration is 3M), and copper foil (2×2cm 2) soaked in hydrochloric acid solution for 24 hours to remove the oxide layer on the surface of the copper foil, and then rinse the copper foil treated with hydrochloric acid with deionized water and methanol in sequence. Weigh 6.5g of 2-methylimidazole and 0.44g of iron acetylacetonate and disperse them in 80mL of methanol solution and keep stirring, then add 2.9g of copper foil after pickling to the above solution and sonicate for 1 hour to obtain A liquid. Next, 6.0 g of Zn(NO 3 ) 2 ·6H 2 O was dissolved in 40 mL of methanol to obtain liquid B. Then pour liquid B into liquid A quickly and stir at 25°C for 24 hours, centrifuge to collect the precipitate of copper-iron bimetallic metal-organic framework precursor, wash with methanol three times and dry in an oven at 60°C to obtain the pr...
Embodiment 2
[0033] In this example, a copper-iron bimetallic confined nitrogen-doped carbon nanotube composite material was prepared in the same manner as in Example 1, except that the pyrolysis temperature was 1050°C.
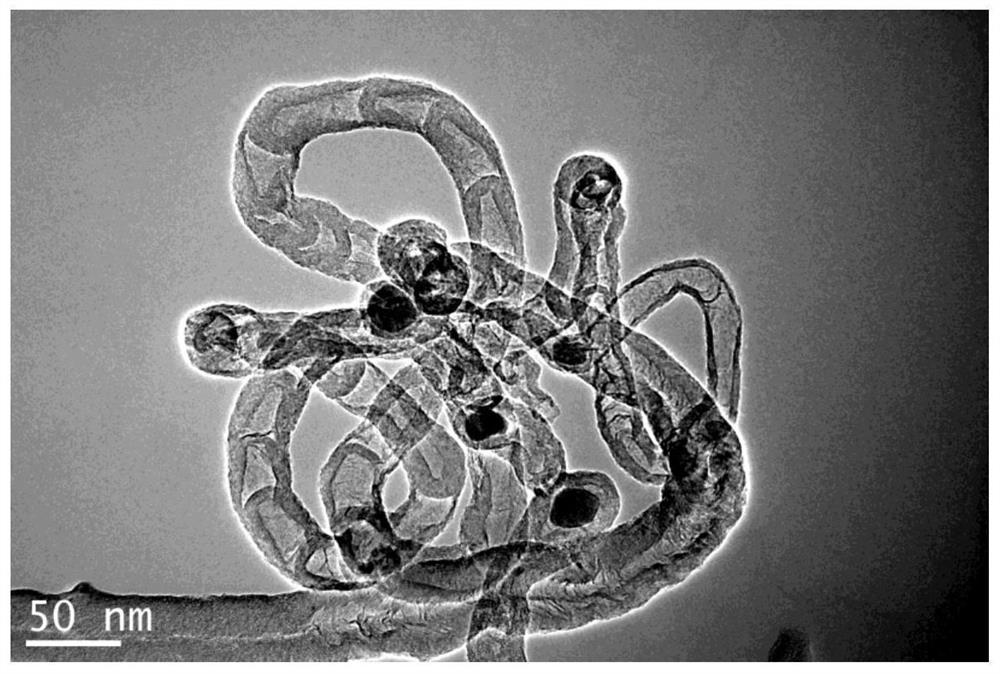
[0034] After testing, the composite material prepared in this embodiment has a carbon nanotube structure, and the bimetallic copper and iron are highly dispersed in the metal-organic framework-derived nitrogen-doped carbon nanotubes, and have high catalytic activity.
[0035] The same method as in Example 1 was used to test the catalytic performance of the composite material prepared in this example. After testing, the degradation rate of organic pollutant Golden Orange II and heavy metal pollutant hexavalent chromium reached 100%.
Embodiment 3
[0037] In this example, a copper-iron bimetallic confined nitrogen-doped carbon nanotube composite material was prepared in the same manner as in Example 1, with the only difference being that the amount of methanol used in liquid A was 40 mL, and the amount of methanol in liquid B was 20 mL during the preparation of the precursor.
[0038] After testing, the composite material prepared in this embodiment has a carbon nanotube structure, and the bimetallic copper and iron are highly dispersed in the metal-organic framework-derived nitrogen-doped carbon nanotubes, and have high catalytic activity.
[0039] The same method as in Example 1 was used to test the catalytic performance of the composite material prepared in this example. After testing, the degradation rate of organic pollutant Golden Orange II and heavy metal pollutant hexavalent chromium reached 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com