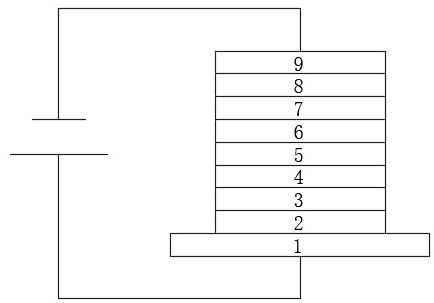
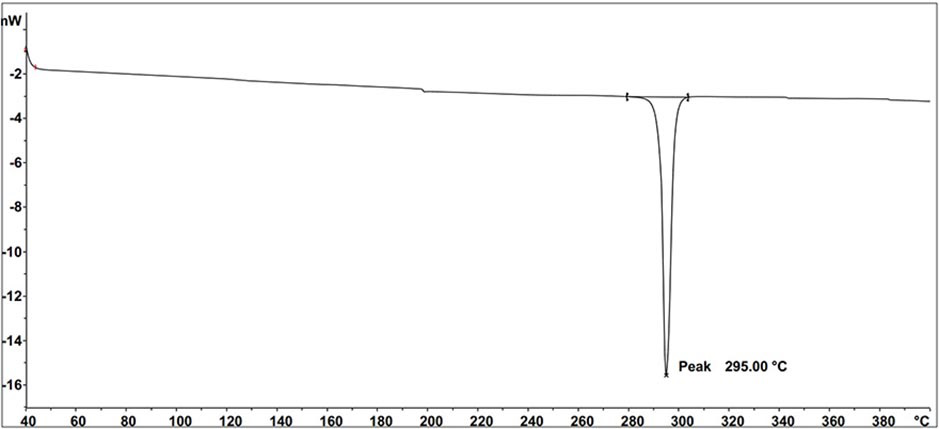
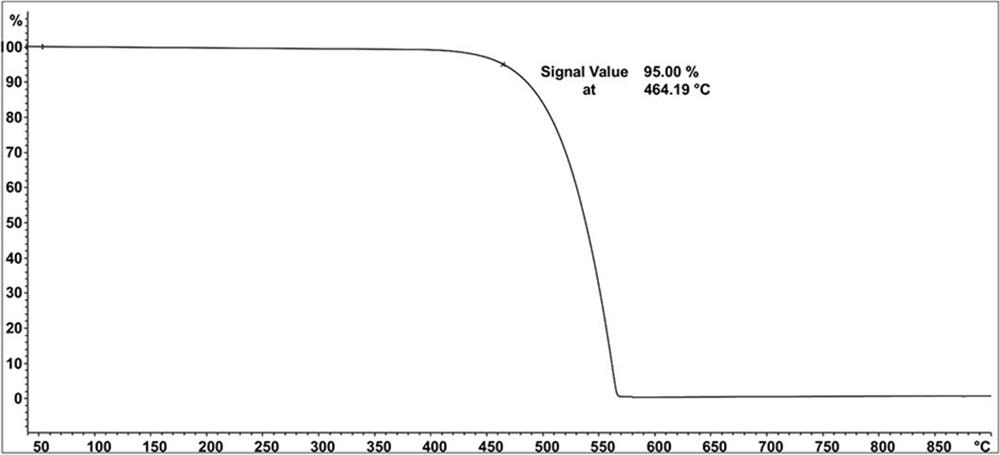
Compound containing triazine and carbazole structure and organic electroluminescent device
A compound, carbazole technology, applied in the field of organic electroluminescence, can solve the problems that restrict the development and application of OLED, and achieve the effect of good luminous efficiency and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148]
[0149] The synthetic method of compound 1 is as follows:
[0150]
[0151] Under nitrogen protection, 1-a (50g, 127mmol, 1eq), 1-b (39.2g, 134mmol, 1.05eq), potassium carbonate (44g, 318mmol, 2.5eq), Pd(PPd3 )4 (2.94g, 2.54mmol, 0.02eq), 400mL of toluene, 200mL of ethanol, and 120mL of water. After the addition, the temperature was raised to 105°C and the reaction was stirred. The reaction progress was monitored by HPLC. When the compound 1-a ≤ 1%, the reaction was stopped. Add 200mL of water, cool down and stir to crystallize. After cooling down to room temperature, suction filter to obtain a filter cake. The filter cake is air-dried at 85°C for 12 hours to obtain 46.3g of gray solid 1-c, ESI-MS (m / z) (M+): theoretical value 409.48, measured value 409.12, elemental analysis results (molecular formula C30H19NO): theoretical value C, 88.00; H, 4.68; N, 3.42; O, 3.91; measured value C, 87.91; H, 4.69; N, 3.50; O, 3.90.
[0152]Under the protection of nitrogen, 1...
Embodiment 2
[0154]
[0155] The synthetic method of compound 2 is as follows:
[0156]
[0157] The preparation method is basically the same as in Example 1, the only difference is that 1-c in Example 1 is replaced by 2-c to obtain the final target product compound 2 with a yield of 46.4%, ESI-MS (m / z) (M+) : theoretical value 645.76, measured value 645.31, elemental analysis results (molecular formula C45H23D5N4O): theoretical value C, 83.70; H, 5.15; N, 8.68; O, 2.48; measured value C, 83.78; H, 5.09; N, 8.57; O , 2.56.
Embodiment 3
[0159]
[0160] The synthetic method of compound 3 is as follows:
[0161]
[0162]
[0163] Under nitrogen protection, in a 2L three-necked flask, 3-a (60g, 244mmol, 1eq), 3-b (42g, 269mmol, 1.1eq), tetrakistriphenylphosphine palladium (4.2g, 3.6mmol, 0.015 eq), potassium carbonate (85g, 615mmol, 2.75eq), 500mL toluene, 125mL ethanol and 125mL water, after the addition, the temperature was raised to 85°C and the reaction was stirred, and the reaction progress was monitored by HPLC. When the compound 3-a ≤ 1%, the reaction was stopped , after the reaction solution was naturally cooled to room temperature, 200mL of water was added, stirred for 10min, filtered with suction, washed with water, rinsed with ethanol, and dried at 85°C for 12 hours to obtain 62.9g of intermediate 3-c with a yield of 93%, ESI- MS (m / z) (M+): theoretical value 277.75, measured value 277.52, elemental analysis results (molecular formula C18H12ClN): theoretical value C, 77.84; H, 4.35; N, 5.04; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com