P-chiral N-vinyl phosphoramide, chiral phosphoramide compound and preparation method of chiral phosphoramide compound
A technology based on phosphonamides and phosphonamides, which is applied in the field of organic synthetic chemistry, can solve problems such as the unreported catalytic methods of chiral phosphonamides, and achieve the effects of great application value, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047]
[0048] Step 1, the reaction is carried out in a glove box, and under an argon protective atmosphere, [Ir(cod)Cl] is added to a 5ml reaction bottle A equipped with a stirring bar 2 (1.4mg, 2.0mol%), chiral phosphoramide ligand (1.9mg, 4.0mol%), 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) (1.4mg , 10.0mol%), reaction solvent toluene (0.5mL), and reacted at 25°C for 30min to generate an orange catalyst pre-stirred solution;
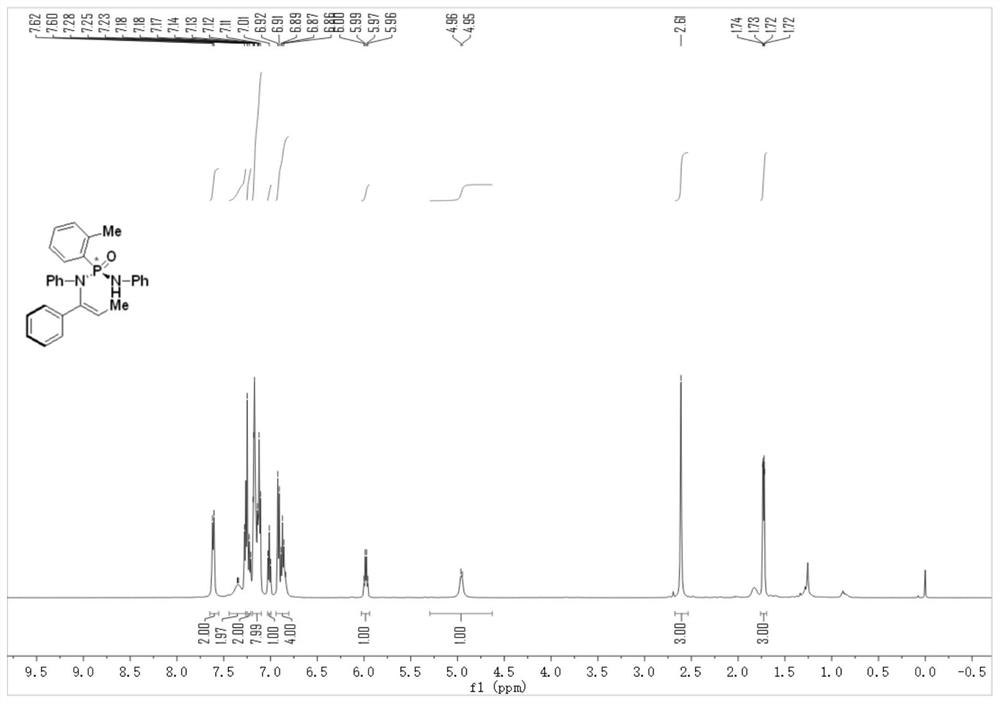
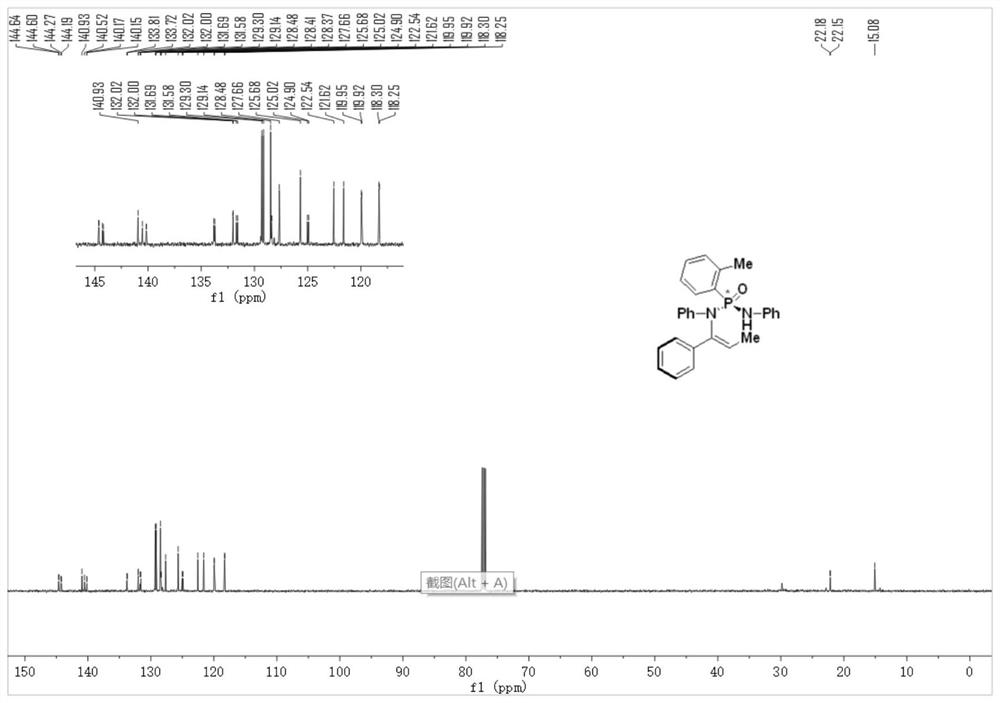
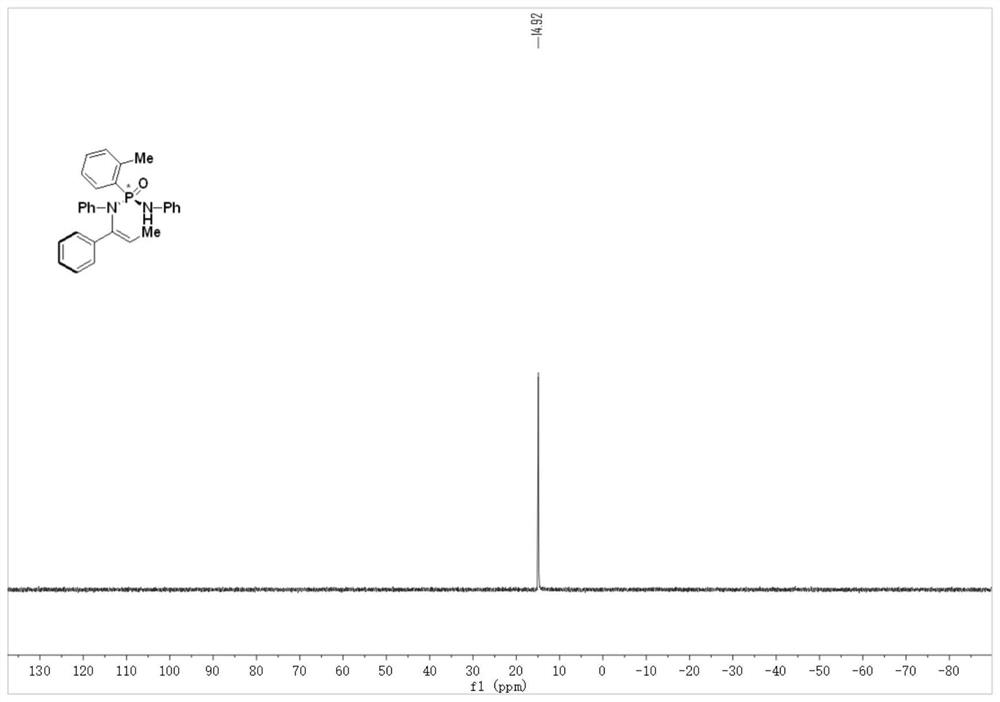
[0049] Step 2, under an argon protective atmosphere, add cinnamyl phosphate (compound 1) (27mg, 1.0eq.), phosphonamide (compound 2) (48mg, 1.5eq.), bis(tri Methylsilyl)potassium amide (KHMDS) (100μL, 1M in THF, 1.0eq.), reaction solvent toluene (0.5mL), and react at 25°C for 48h. After the reaction, the crude product was purified by silica gel column chromatography to obtain P-chiral N-vinylphosphonamide (compound 3), with a yield of 80% and an ee of 91%;
[0050] 1 H NMR (500MHz, Chloroform-d) δ7.61(d, J=8.0Hz, 2H), 7.35–7.34(m, 2H), 7.24(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com