Shampoo for treating less hair and alopecia
A shampoo and hair loss technology, applied in the field of medicine or cosmetics, can solve the problems of insufficient types of medicines and insufficient types of 5α reductase inhibitors, and achieve good application value and the effect of promoting hair growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Screening of Type II 5α Reductase High Immunogens
[0039] According to the bioinformatics analysis of the amino acid sequence of human and mouse type II 5α reductase, the secondary structure, antigenicity, hydrophilicity, accessibility and flexibility of PCT protein were comprehensively analyzed, and each segment was analyzed and scored. The high-scoring region was used as the B-cell epitope region. Specifically, using technical parameters such as prediction of β-turn angle by Chou&Fasman method, prediction of antigen surface accessibility by Emini method, prediction of protein flexibility by Karplus&Schulz method, protein antigenicity analysis by Kolaskar&Tongaonkar method, protein hydrophobicity analysis by Parker method and Bepipred linear epitope prediction and other technical parameters, type II was obtained. The epitope peptide of 5α reductase; the chemically synthesized epitope peptide is: N-cvsganflgeiiew-C (SEQ ID NO: 1), and cysteine is introduced...
Embodiment 2
[0040] Example 2 Preparation of Type II 5α Reductase Monoclonal Antibody
[0041] For the first immunization, the immunogen synthesized in Example 1 was diluted to 1 mg / ml with 0.01M pH7.4 phosphate buffer, emulsified and mixed with complete Freund's adjuvant, 50 μg of immunogen per mouse. Inoculate by subcutaneous injection in abdomen and back of mice. Every 2 to 3 weeks, the same amount of immunogen was emulsified and mixed with Freund's incomplete adjuvant for booster immunization, a total of 4 times of immunization, and 1 week after the last immunization, blood was collected from the orbit of the immunized mice, and the serum was separated by centrifugation. In the indirect ELISA method, the immunogen is used to coat the microtiter plate, and the immune titer is detected respectively. The mice with relatively high immune titer were screened out, and 100 μg immunogen was injected intraperitoneally on the 10th day of the last immunization for shock immunization, and routine...
Embodiment 3
[0045] Example 3 Antibody identification
[0046] (1) Affinity identification
[0047] Antibody affinity refers to the tightness of the binding of the antibody to the antigenic determinant. The stronger the affinity, the stronger the binding. It is one of the most important indicators for evaluating the properties of antibodies. Using the FortebioOctetK2 instrument of PALL Company, the affinity constant of the antibody was determined by a kinetic method. The result is that the affinity constant of the antibody is 7.2×10-10, which has a good affinity effect.
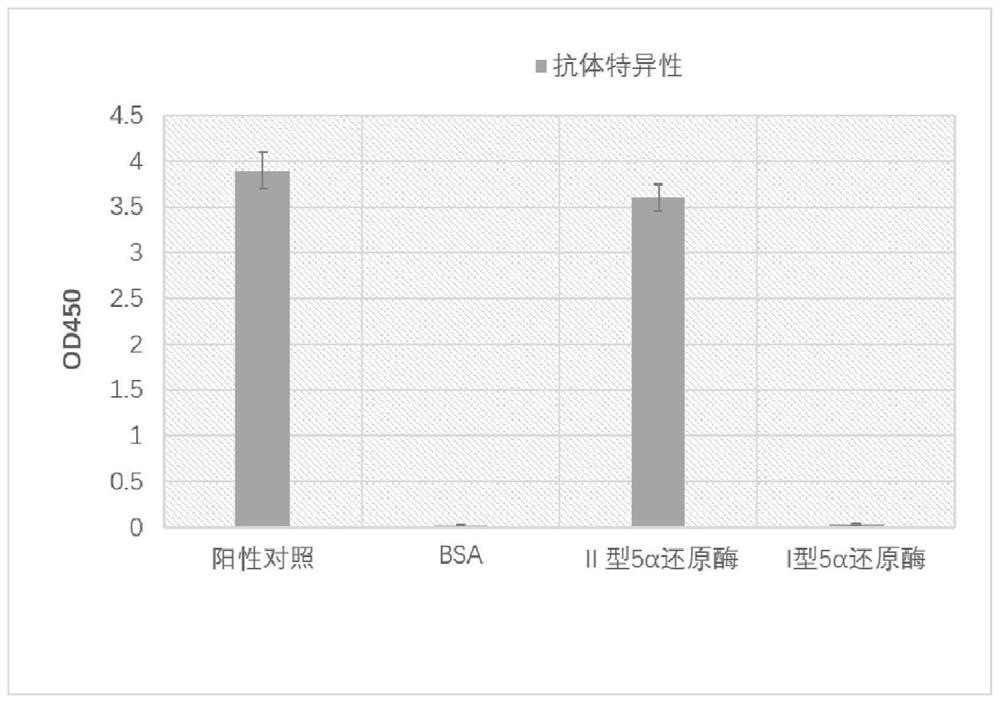
[0048] (2) Monoclonal antibody specific detection
[0049] The specificity of monoclonal antibody was detected by ELISA indirect assay method, and the immunogenic peptide was used as positive control, and the specificity of monoclonal antibody was identified with 2 μg / ml BSA, type II 5α reductase, and type I 5α reductase. The result is as figure 1 shown.
[0050] From figure 1 It can be seen from the results that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com