Bifunctional antibody for relieving immunosuppression in tumor immune microenvironment as well as application and preparation method of bifunctional antibody
A bifunctional antibody, immunosuppressive technology, applied in chemical instruments and methods, biochemical equipment and methods, antibodies, etc., can solve problems such as the disadvantage of tumor treatment effect, and achieve improved targeting and safety, efficient and safe selection. , the effect of enhancing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
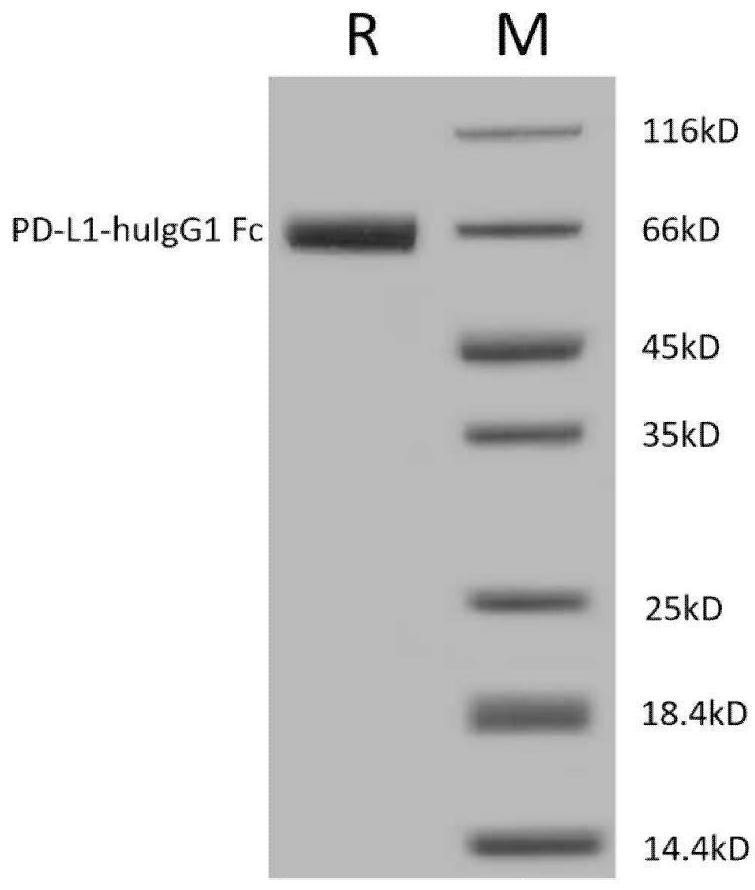
[0049] This example constructs the recombinant PD-L1-huIgG1 Fc fusion protein, and its specific method is as follows:
[0050] 1. Synthesize the gene sequence of the 19th to 238th amino acid interval of PD-L1 and construct the expression vector of PD-L1-huIgG1 Fc fusion protein
[0051] The gene sequence from the 19th phenylalanine to the 238th arginine of human programmed death ligand-1 (PD-L1) (NCBI accession NP_054862) was synthesized by chemical synthesis, and the amino acid encoded by the gene sequence 序列为FTVTVPKDLYVVEYGSNMTIECKFPVEKQLDLAALIVYWEMEDKNIIQFVHGEEDLKVQHSSYRQRARLLKDQLSLGNAALQITDVKLQDAGVYRCMISYGGADYKRITVKVNAPYNKINQRILVVDPVTSEHELTCQAEGYPKAEVIWTSSDHQVLSGKTTTTNSKREEKLFNVTSTLRINTTTNEIFYCTFRRLDPEENHTAELVIPELPLAHPPNER(SEQID NO:1)。 Synthesize the gene sequence from the 100th proline to the 330th lysine of the human IgG1 heavy chain constant region (UniProtKB / Swiss-Protaccession No.P01857.1) by chemical synthesis, the amino acid sequence encoded by the gene sequence 为PKS...
Embodiment 2
[0055] This example provides an anti-human PD-L1 murine antibody, the preparation method of which is as follows:
[0056] 1. Immunization of animals
[0057] 2 mg / mL of the above recombinant PD-L1-huIgG1 Fc fusion protein was mixed and emulsified as an antigen with an equal volume of complete Freund's adjuvant (Sigma-Aldrich), and 10 6-week-old female Balb / c mice were subcutaneously immunized. After the initial immunization, a booster immunization was carried out every ten days, and a total of four subcutaneous immunizations were performed. In the fifth immunization, the spleen was directly immunized with MSLN-huIgG1Fc fusion protein.
[0058] 2. Serum titer detection
[0059] Before each booster immunization, 50 μL of blood was collected from the tail vein, and the cells were removed by centrifugation, and the serum was retained. Add recombinant PD-L1 50ng / well to the ELISA microwell plate, and coat overnight at 4°C. Wash three times with PBS, add 1% BSA / PBS, 200 μL / well, ...
Embodiment 3
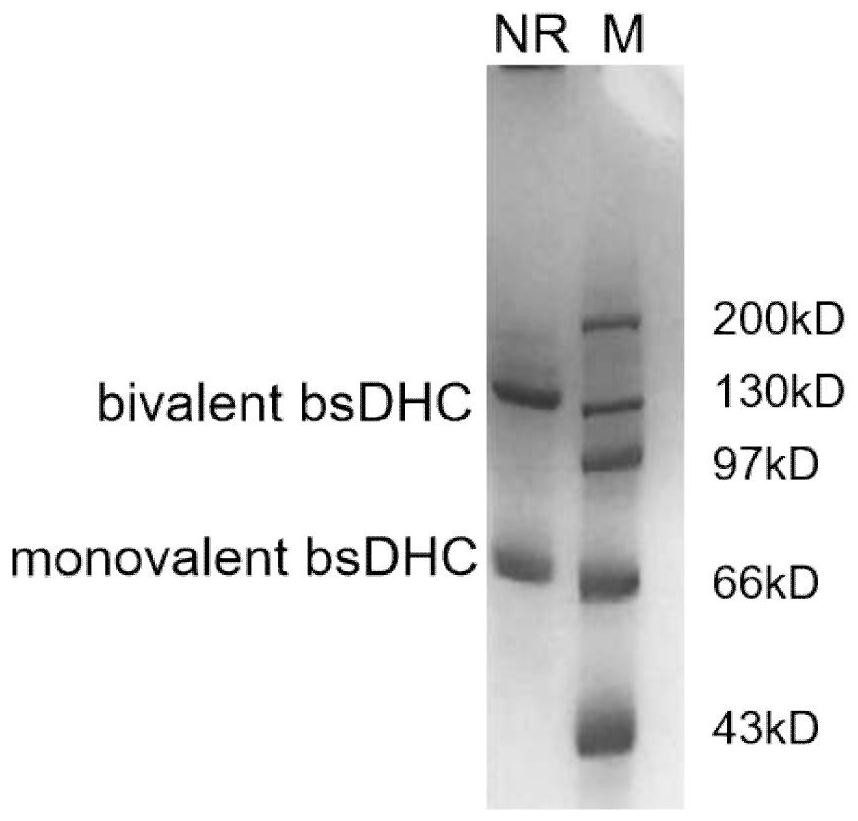
[0115] Based on Example 2, this example provides a bifunctional antibody that relieves immunosuppression in the tumor immune microenvironment. The design and preparation process are as follows:
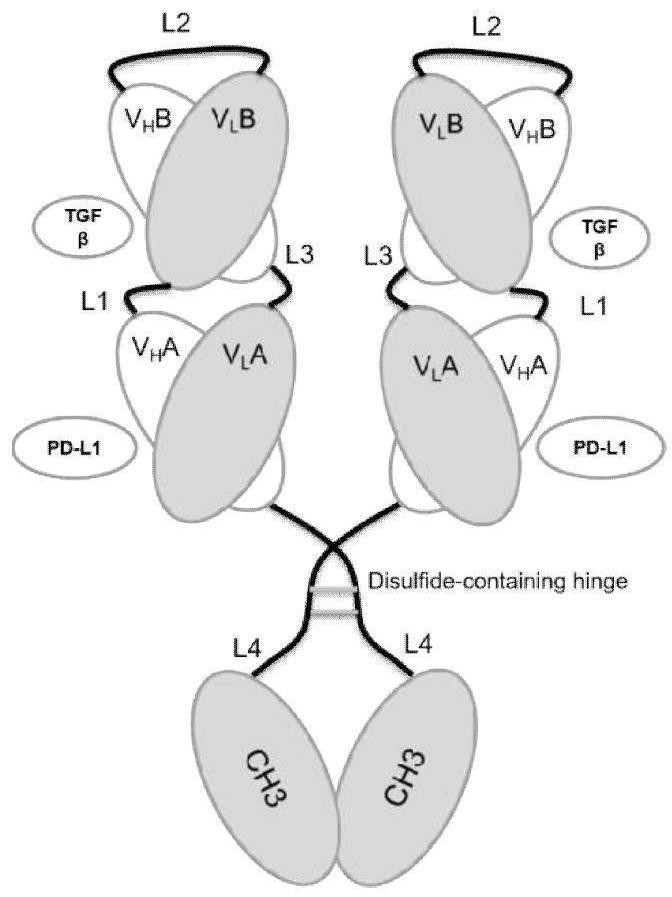
[0116] 1. Structural design of bifunctional antibody
[0117] Design a bifunctional scDb (single chain diabody) that is stabilized by a disulfide bond formed by the combination of the variable region of the anti-PD-L1 antibody and the variable region of the TGF-βs antibody (formation of a disulfide bond through the hinge region containing cysteine)- The CH3 structure (hereinafter referred to as bsDHC, bispecific scDb-Hinge-CH3), specifically consists of the light chain variable region of the PD-L1 antibody, the L1 short connecting peptide, the heavy chain variable region of the TGF-β antibody, the L2 long connecting peptide, Light chain variable region of TGF-β antibody, L3 short connecting peptide, heavy chain variable region of PD-L1 antibody, hinge domain of human IgG 1 that can ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com