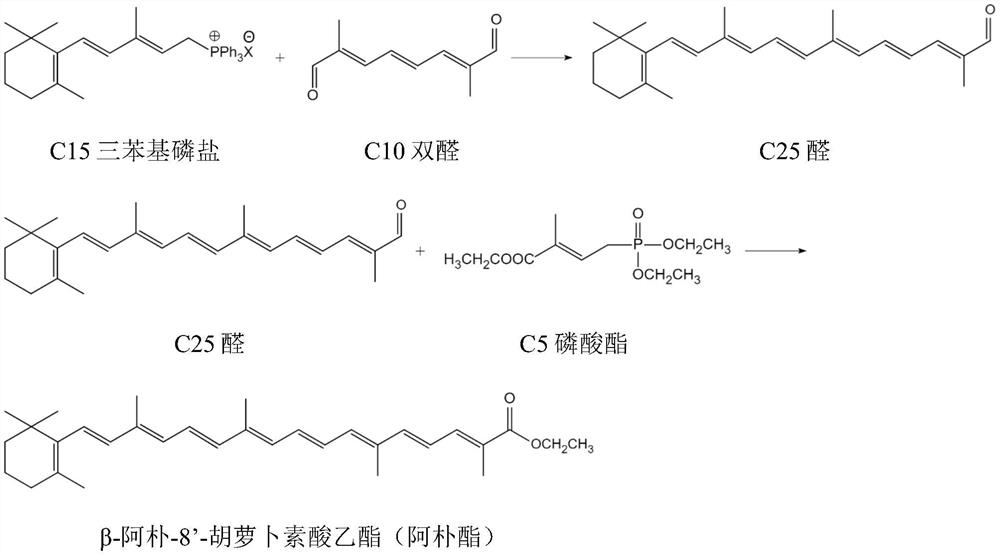
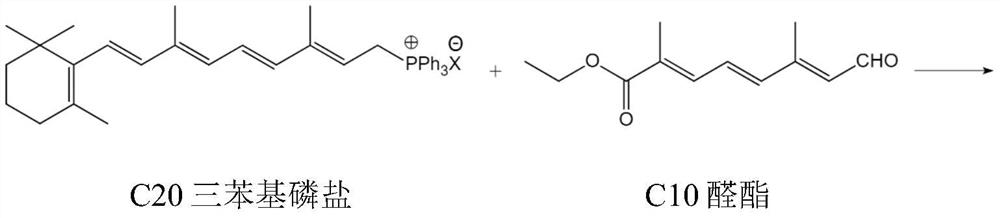
Synthetic method of beta-apo-8'-carotenoic ethyl ester
A carotene and synthesis method technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of difficult handling and high cost of raw materials, and achieve fewer purification steps, lower production costs, and difficulty in industrialization. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
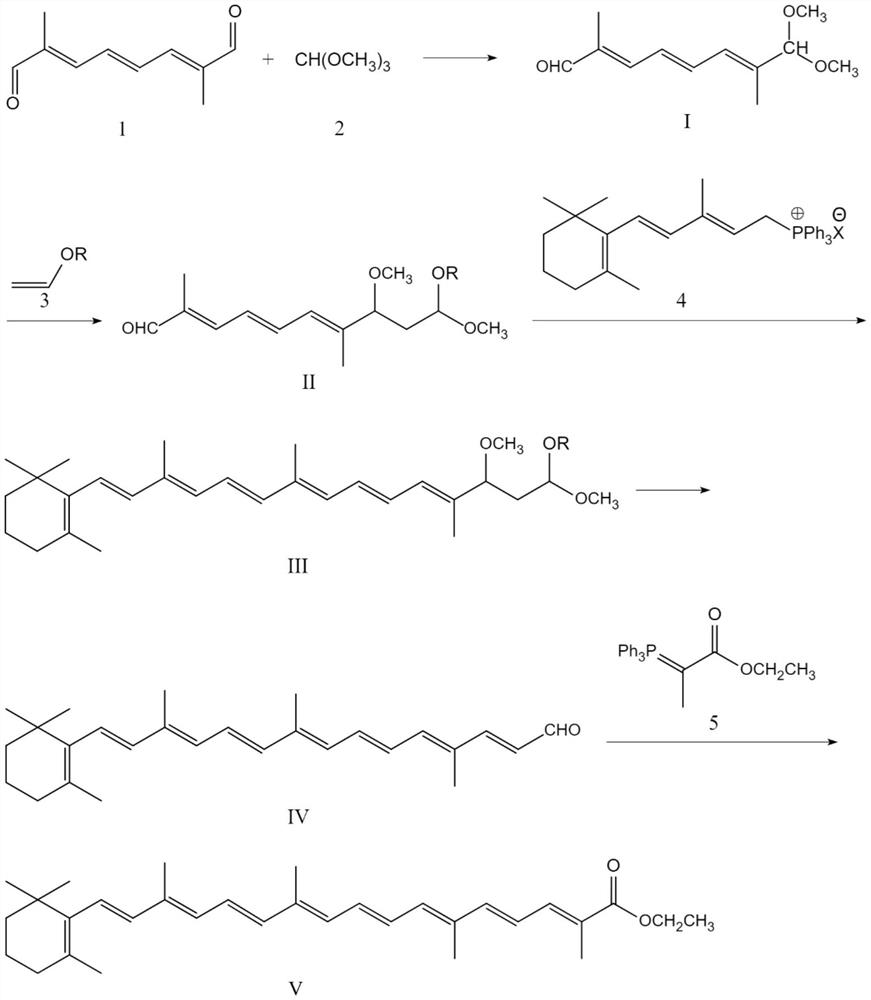
[0039] The method for synthesizing β-apo-8'-carotene acid ethyl ester according to the above-mentioned route comprises the steps:
[0040] S1. Synthesis of compound I
[0041]
[0042] Dissolve 16.4g (0.1 mole) of C10 dialdehyde in 120ml of dichloromethane, then add 7.42g (0.07 mole) of trimethyl orthoformate, 0.3 g of p-toluenesulfonic acid to catalyze the reaction, and maintain the reaction temperature at 30-35°C Reaction, follow the reaction with the liquid phase, the increase in the product content in half an hour is between 0.3% and 0.5%, which is the end point, then add 0.1g sodium methoxide to neutralize the reaction, wash with 40ml×2 water, and then recover dichloromethane under reduced pressure. After recycling, add 200ml of absolute ethanol to reflux for half an hour, cool to room temperature, filter to obtain 4.1g of unreacted C10 bisaldehyde, and recover ethanol from the mother liquor to obtain compound I. The yield was calculated to obtain 14.3 g of the target...
Embodiment 2
[0056] S1. Synthesis of compound I
[0057]
[0058] Dissolve 16.4g (0.1 mole) of C10 dialdehyde in 120ml of dichloromethane, then add 5.3g (0.05 mole) of trimethyl orthoformate, 0.3 g of p-toluenesulfonic acid to catalyze the reaction, and maintain the reaction temperature at 30-35°C Reaction, follow the reaction with the liquid phase, the increase in the product content in half an hour is between 0.3% and 0.5%, which is the end point, then add 0.1g sodium methoxide to neutralize the reaction, wash with 40ml×2 water, and then recover dichloromethane under reduced pressure. After recycling, add 200ml of absolute ethanol to reflux for half an hour, cool to room temperature, filter to obtain 8.6g of C10 dialdehydes that have not participated in the reaction, and recover ethanol from the mother liquor to obtain compound I. The yield was calculated to obtain 9.3 g of the target product with a content of 96.2% and a yield of 93.1%.
[0059] S2. Synthesis of Compound II
[0060...
Embodiment 3
[0072] S1. Synthesis of compound I
[0073]
[0074] Dissolve 16.4g (0.1 mole) of C10 dialdehyde in 120ml of dichloromethane, then add 10.6g (0.1 mole) of trimethyl orthoformate, 0.3 g of p-toluenesulfonic acid to catalyze the reaction, and maintain the reaction temperature at 30-35°C Reaction, follow the reaction with the liquid phase, the increase in the product content in half an hour is between 0.3% and 0.5%, which is the end point, then add 0.1g sodium methoxide to neutralize the reaction, wash with 40ml×2 water, and then recover dichloromethane under reduced pressure. After recycling, add 200ml of absolute ethanol to reflux for half an hour, cool to room temperature, filter to obtain 10g of unreacted C10 bisaldehyde, recover ethanol from the mother liquor to obtain compound I, and calculate according to the actual consumed C10 bisaldehyde (actual feeding-recovered raw material) Yield: 7.1 g of the target product was obtained with a content of 96.5% and a yield of 86.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com