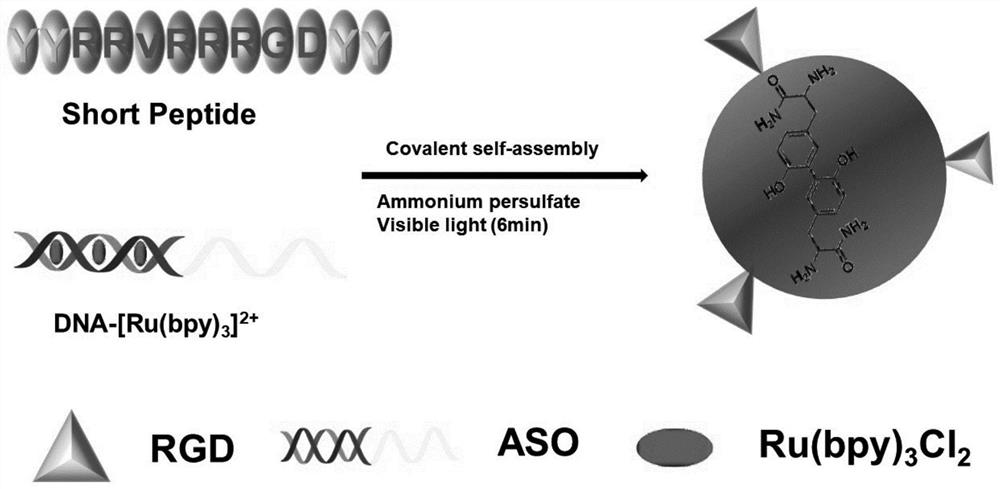
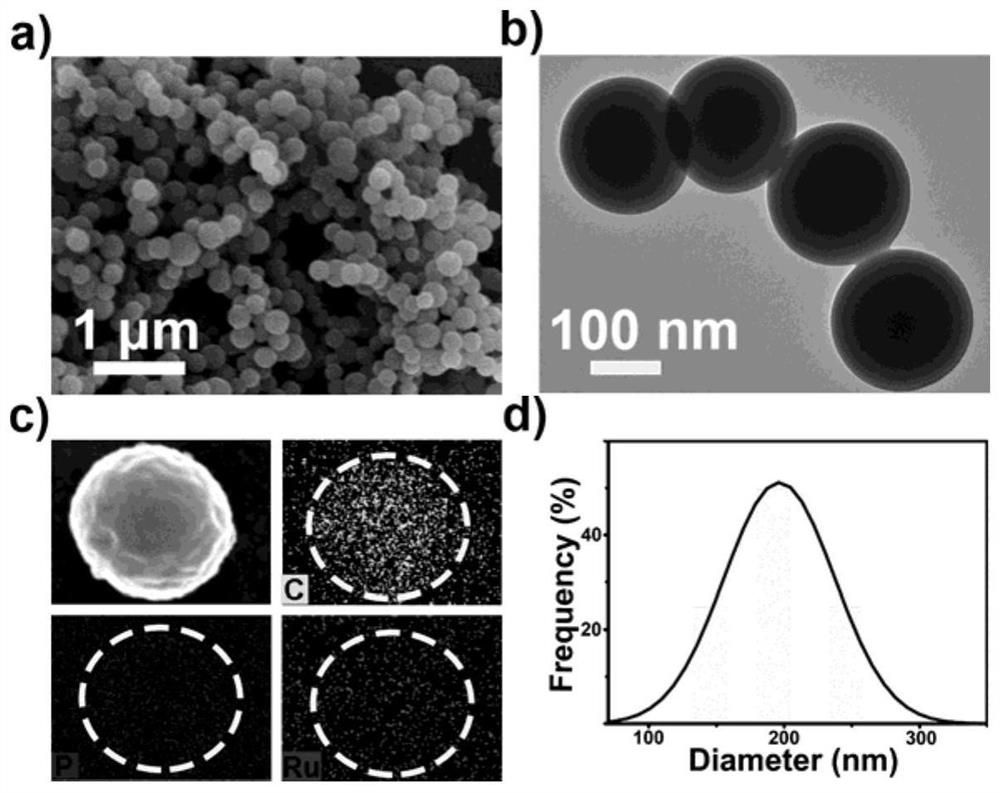
Non-viral vector formed based on covalent assembly of terpyridyl ruthenium catalytic oligopeptides, and preparation method and application of non-viral vector
A non-viral carrier, terpyridine ruthenium chloride technology, applied to the preparation method of peptides, chemical instruments and methods, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A DNA-[Ru(bpy) based 3 ] 2+ A preparation method for catalyzing the covalent assembly of short peptides to form a non-viral vector, the preparation method comprising the following steps:
[0039](1) Heat equal volumes of DNA S1 solution and DNA S2 solution with a concentration of 2 μM to 90°C, take it out after 10 minutes, cool to room temperature, and then store in the upper layer of the refrigerator at a temperature of 4-10°C until the dsDNA structure is formed, Obtain dsDNA solution;
[0040] The gene sequence of the DNA S1 is: 3'-GGA TTG GAG TTC CTC CAG CGT GCG CCA TCC TTC CCA TCCTCC TCC -5'; the underlined part is the ASO sequence that can reduce the expression of the anti-apoptotic protein Bcl-2. If it is replaced with a nucleic acid sequence that can treat other diseases, the targeted treatment of other diseases can also be achieved;
[0041] The gene sequence of the DNA S2 is: 3'-GAG GAA CTC CAA TCC-5';
[0042] (2) 1mL 2mM [Ru(bpy) 3 ] Cl 2 The solut...
Embodiment 2
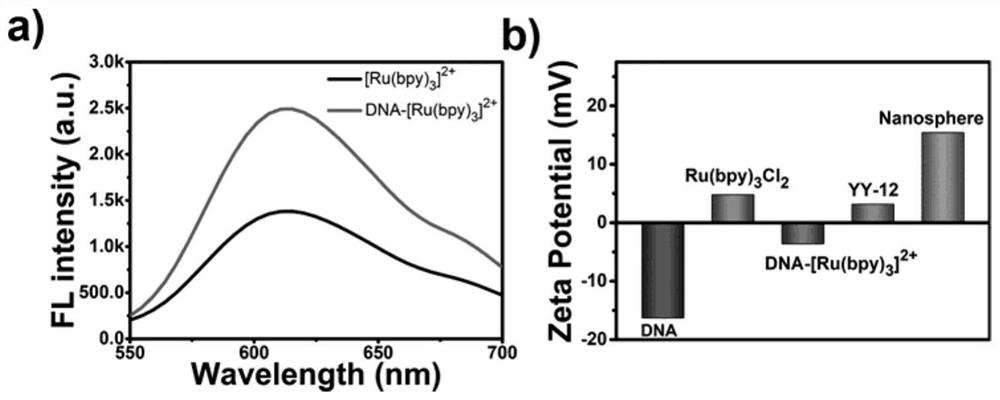
[0046] Others are with embodiment 1, test [Ru(bpy) when carrying out step (2) 3 ] Cl 2 , DNA-[Ru(bpy) 3 ] 2+ fluorescence spectrum, such as image 3 As shown in a), generate DNA-[Ru(bpy) 3 ] 2+ When the 625nm characteristic fluorescence peak intensity increases, it proves that DNA-[Ru(bpy)3] 2+ The formation of small molecule; Carry out the measurement of Zeta potential when carrying out step (2-4) simultaneously, as image 3 As shown in b), when [Ru(bpy) 3 ] Cl 2 dsDNA solution was added to the solution to prepare DNA-[Ru(bpy) 3 ] 2+ , DNA-[Ru(bpy) 3 ] 2+ The absolute value of the Zeta potential of dsDNA becomes smaller than that of dsDNA, which is due to [Ru(bpy) 3 ] 2+ With a positive charge, it is further proved that [Ru(bpy) 3 ] 2+ Successful insertion in dsDNA yields DNA-[Ru(bpy) 3 ] 2+ ; when DNA-[Ru(bpy) 3 ] 2+ After mixed with YY-12 and irradiated with light to obtain a non-viral vector, the zeta potential was further corrected, which may be due to ...
Embodiment 3
[0048] Others are the same as in Example 1, when carrying out step (4), test the fluorescence spectrum of YY-12 and the non-viral vector obtained by the reaction, such as image 3 As shown in a), the fluorescence peak of the generated peptide-nucleic acid nanospheres is 410nm, which proves the formation of non-viral vectors; after the reactants are mixed evenly, the ultraviolet absorption diagrams of the test system are sampled under different irradiation times, and the test time is cut off at 600s, 6min Afterwards, the peak value tends to be flat and no longer rises, and the UV absorbance value at this time reaches the maximum, which also proves the success of the covalent cross-linking of the short peptide and the successful preparation of a non-viral vector.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap