Lentiviral vector expression system for polygene transformation
A lentiviral vector and expression system technology, applied in the direction of virus/bacteriophage, reverse transcription RNA virus, introduction of foreign genetic material using vectors, etc., can solve the problems of harsh cells, achieve the effect of improving the expression rate and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Construction of the first and second lentiviral vectors
[0081] 1. Construction of vectors pLent-EF1a-MCS-CMV-N-Puro-TS1 and pLent-EF1a-MCS-CMV-TS2-C-Puro
[0082] 1. Design primers. See Table 3 for specific primers:
[0083] Table 3: PCR Primers
[0084]
[0085] 2. PCR, obtain N-Puro-TS1 fragment and TS2-C-Puro fragment by PCR technique.
[0086] Table 4: PCR system: (unit: μL)
[0087]
[0088] Reaction procedure:
[0089] Table 5: PCR program
[0090]
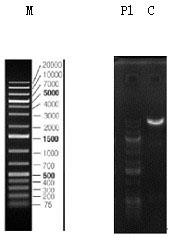
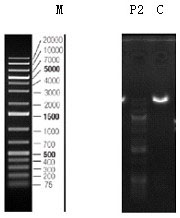
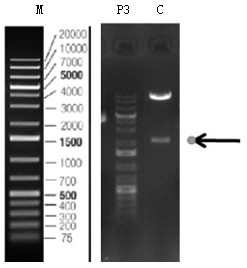
[0091] After the PCR reaction, take 2 µl of the PCR product and use 1.5% agarose gel electrophoresis to separate and detect it. If the size of the target band is correct, use the PCR purification recovery kit to recover the target fragment.
[0092] 3. Digestion
[0093] (1) Digest the N-Puro-TS1 and TS2-C-Puro obtained by PCR, and screen the enzyme digestion system as shown in Table 6:
[0094] Table 6: Enzyme digestion system for screening genes
[0095]
[0096] (2) After adding th...
Embodiment 2
[0157] Example 2: Vector transfected cells, target gene transcription
[0158] The constructed pLent-EF1a-Oct4-P2A-Sox2-CMV-N-Puro-TS1 (P3) vector and pLent-EF1a-Klf4-P2A-Myc-CMV-TS2-C-Puro (P4) vector were co-transfected into HEK293 Cells, puro screening for 72 hours of viability verification and detection of mRNA levels of four genes
[0159] 1. The constructed pLent-EF1a-Oct4-P2A-Sox2-CMV-N-Puro-TS1 vector and pLent-EF1a-Klf4-P2A-Myc-CMV-TS2-C-Puro vector were co-transfected into HEK293 cells, puro screening 72 hours of viability verification
[0160] 1.1 Normal-growing HEK293 cells were spread in 6-well plates;
[0161] 1.2 Carry out transfection experiments according to Table 17:
[0162] 1.2.1 Cell culture: take 6-well plate cell culture dish, add 2mL containing 1~2×10 5 cell culture medium at 37°C CO 2 The culture density is 40%~60%, if the density is too high, it is not easy to select cells after transfection.
[0163] 1.2.2 Preparation of transfection solution A...
Embodiment 3
[0186] Example 3: Infection of 293A cells with packaging lentivirus and selection of stably transfected cells
[0187] 3.1 Packaging lentiviral products:
[0188] 3.1.1 Spread HEK293T cells in a 10cm dish, and the next day the cell confluency reaches 85%-90%;
[0189] 3.1.2 Configure the transfection reagent according to the ratio, mix it and let it stand at room temperature for 3 minutes, then add PEI and vortex to mix, let it stand at room temperature for 30 minutes, add it to a 10cm cell culture dish, and replace it with fresh medium 6 hours after transfection;
[0190] 3.1.3 Observe the transfection efficiency and take pictures;
[0191] 3.1.4 Collect the supernatant medium after 72 hours of transfection, pass through a 0.45um filter membrane; put the filtered supernatant medium into an ultrafiltration tube for ultracentrifugation, 25000rpm, 4°C, 1.5hs;
[0192] 3.1.5 Collect viruses.
[0193] 3.2 Detection of virus titer:
[0194] 3.2.1 6 hours before infection in 24-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com