Deuterated 2-substituted aniline-4-indolyl pyrimidine derivative as well as preparation method and application thereof
A technology of indolylpyrimidine and its derivatives, which is applied in the field of medicine, can solve the problem of EGFR selectivity reduction, and achieve high activity, increased metabolic stability, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
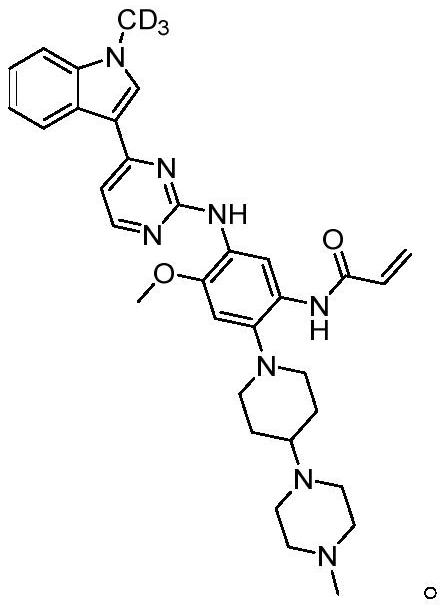
[0027] This embodiment provides a compound VIII-1, the structural formula of the compound VIII-1 is:
[0028]
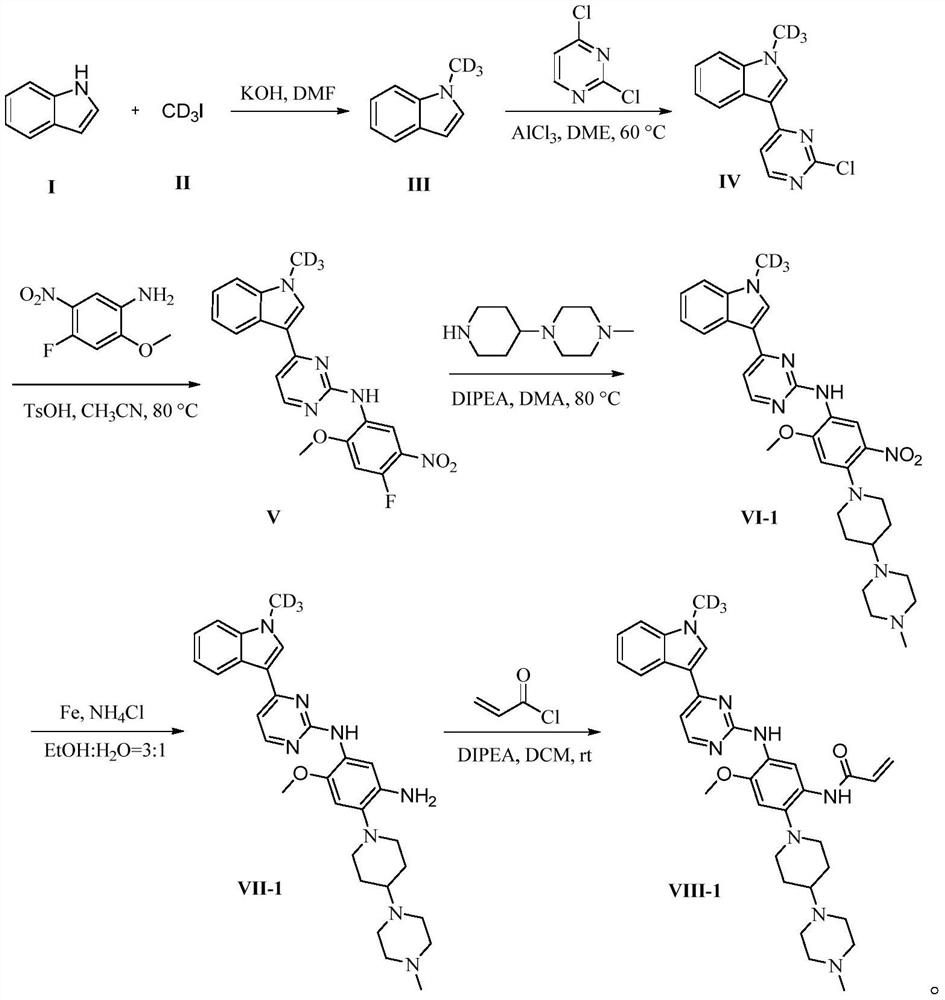
[0029] The compound VIII-1 is synthesized according to the following steps:
[0030]
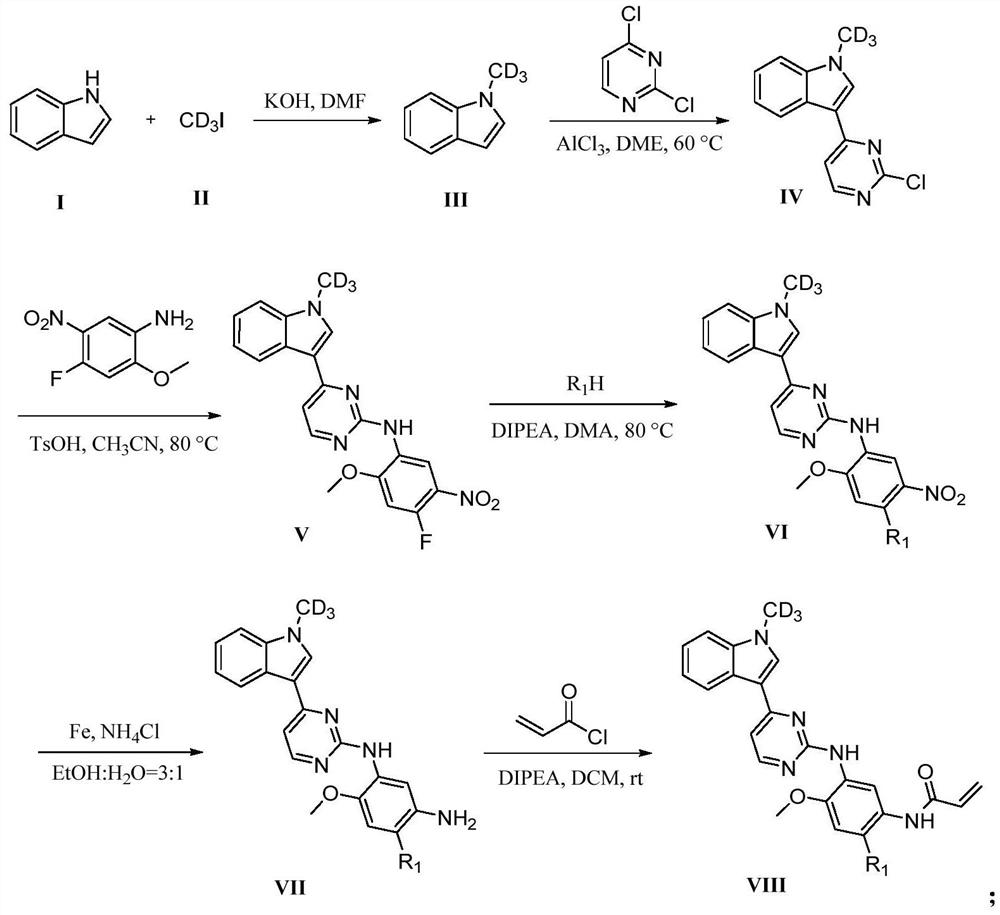
[0031] Specifically, the preparation method of compound VIII-1 comprises the following steps:
[0032] Preparation of Intermediate III Add 5.00g (42.68mmol) indole I and 7.28g (128.04mmol) KOH into 50mL DMF, stir at 0°C for 30min, then slowly add 7.42g (51.22mmol) deuteromethyl iodide II dropwise To the above system, stirred at room temperature for 12h. The reaction was monitored by thin-layer chromatography TLC. After the reaction was completed, the reaction system was extracted with ethyl acetate (3×150mL), the organic phases were combined, and the organic phases were washed with anhydrous MgSO 4 After drying, filter with suction, and concentrate Intermediate III under reduced pressure to obtain 5.121 g of light yellow liquid with a yield of 89%. 1 H NMR (400MHz, DMSO-d ...
Embodiment 2
[0039] This embodiment provides a compound VIII-2, the structural formula of the compound VIII-2 is:
[0040]
[0041] This example also provides a preparation method of compound VIII-2, which is basically the same as the preparation method of compound VIII-1 provided in Example 1, the difference mainly lies in: the intermediate VI-2, intermediate The structural formula of body VII-2 is different from that in Example 1, especially the group R in the structural formula of intermediate VI-2 and intermediate VII-2 1 different, the group R in this example 1 for:
[0042] Compound VIII-2 prepared by the above method is a white solid, and the calculated yield is about 90%; 1 H NMR (400MHz, DMSO-d 6 )δ9.04(s,1H),8.80(s,1H),8.57(s,1H),8.31(d,J=5.3Hz,1H),8.26(d,J=7.7Hz,1H),7.87( s,1H),7.52(d,J=8.1Hz,1H),7.26-7.19(m,2H),7.19-7.14(m,1H),6.84(s,1H),6.71(dd,J=16.9, 10.2Hz, 1H), 6.47(br, 1H), 6.25(d, J=16.9Hz, 1H), 5.75(d, J=10.2Hz, 1H), 3.87(s, 3H), 2.88-2.72(m, 4H), 2.22-2.06 (...
Embodiment 3
[0044] This embodiment provides a compound VIII-3, the structural formula of the compound VIII-3 is:
[0045]
[0046] This example also provides a preparation method of compound VIII-3, which is basically the same as the preparation method of compound VIII-2 provided in Example 2, the main difference being that 0.10 g (0.16 mmol) of compound VIII- 2. Add 0.18g (1.63mmol) trifluoroacetic acid into 2mL dichloromethane, and stir at room temperature for 1h. The reaction was monitored by thin-layer chromatography TLC. After the reaction was complete, the reaction solution was directly concentrated under reduced pressure, and the concentrate was extracted with saturated potassium carbonate aqueous solution and ethyl acetate (3×50mL), and the organic phases were combined, anhydrous MgSO 4 Dry, filter with suction, and concentrate under reduced pressure to obtain compound Ⅷ-3. The compound VIII-3 is a white solid, and the calculated yield is about 99%; 1 H NMR (400MHz, DMSO-d 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com