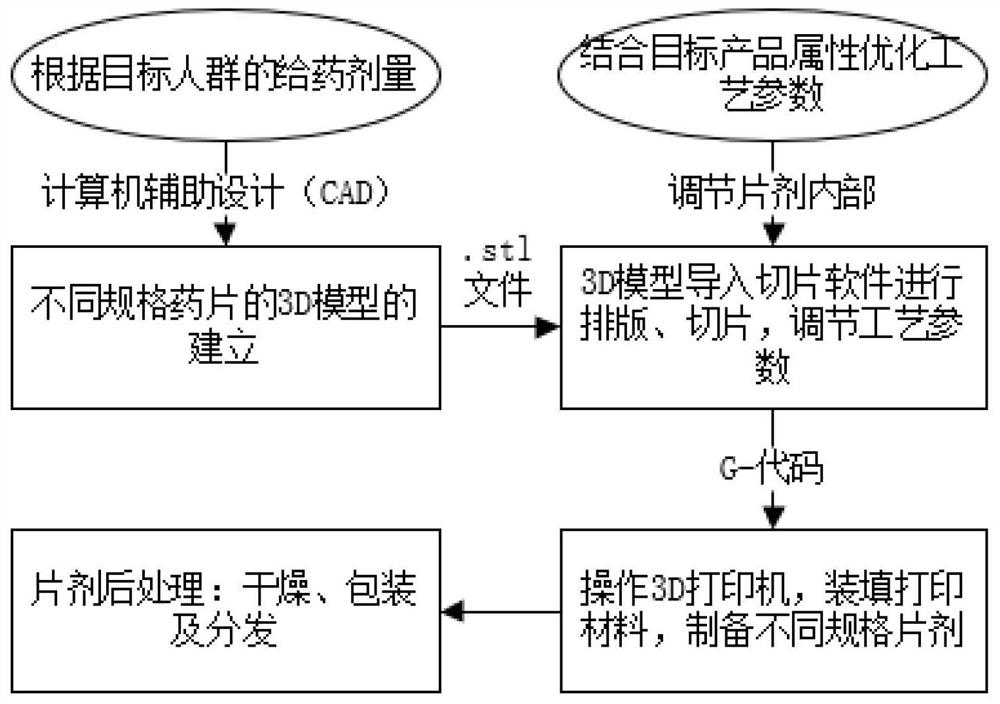
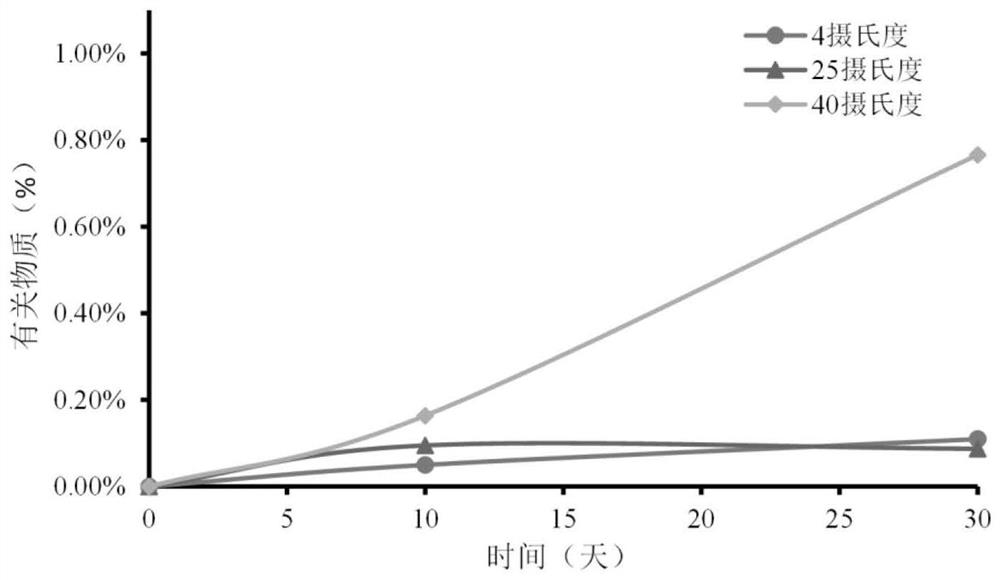
3D printing preparation as well as preparation method and application thereof
A 3D printing and preparation technology, applied in the field of medicine, can solve the problems of insufficient pediatric preparations, uneven dispersion, dosage errors, etc., and achieve the effect of ensuring the safety of clinical medication, good stability, and excellent release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1 Preparation of amlodipine besylate 3D printing preparation
[0143]
[0144]
[0145] The preparation method of amlodipine besylate 3D printing preparation comprises the following steps:
[0146] 1. Preparation of powder mixture
[0147] Mannitol is passed through a 100-mesh sieve, maltitol is crushed and passed through a 100-mesh sieve, amlodipine besylate, mannitol, maltitol, sucralose, sodium carboxymethylcellulose, sodium carboxymethyl starch, tartrazine, Add the lemon essence into the wet mixer, set the stirring rate to 300rpm, and the shear rate to 650rpm, and stir for 10min to make the powder mix uniformly to obtain a powder mixture.
[0148] 2. Liquid mixture preparation
[0149] Add methylparaben into purified water, heat at 80°C for 30min, completely dissolve methylparaben and evenly disperse in purified water, after cooling, add citric acid and glycerin, stir until uniformly mixed, and obtain a liquid mixture.
[0150] 3. Preparation of s...
Embodiment 2
[0167] Example 2 Preparation of amlodipine besylate 3D printing preparation
[0168]
[0169]
[0170] The preparation method of amlodipine besylate 3D printing preparation comprises the following steps:
[0171] 1. Preparation of powder mixture
[0172] Mannitol passed through a 100-mesh sieve, maltitol crushed and passed through a 100-mesh sieve, amlodipine besylate, mannitol, maltitol, sucralose, sodium carboxymethylcellulose, tartrazine and lemon essence were added to the wet mix In the machine, set the stirring rate to 500rpm, the shear rate to 500rpm, and stir for 15min to make the powder mix uniformly and obtain a powder mixture.
[0173] 2. Preparation of liquid mixture
[0174] Add methylparaben to purified water, heat at 70°C for 30min, completely dissolve methylparaben and evenly disperse in purified water, add citric acid and glycerin after cooling, stir and mix evenly to obtain a liquid mixture.
[0175] 3. Preparation of semi-solid materials
[0176] ...
Embodiment 3
[0192] Example 3 Preparation of amlodipine besylate 3D printing preparation
[0193]
[0194]
[0195] The preparation method of amlodipine besylate 3D printing preparation comprises the following steps:
[0196] 1. Preparation of powder mixture
[0197] Mannitol passed through a 100-mesh sieve, maltitol crushed and passed through a 100-mesh sieve, amlodipine besylate, mannitol, maltitol, sucralose, sodium carboxymethylcellulose, crospovidone, tartrazine, lemon The essence was added into a wet mixer, the stirring rate was 400 rpm, the shear rate was 550 rpm, and the stirring rate was 550 rpm for 10 minutes, so that the powder was mixed evenly to obtain a powder mixture.
[0198] 2. Preparation of liquid mixture
[0199] Referring to Example 1.
[0200] 3. Preparation of semi-solid materials
[0201] Referring to Example 2.
[0202] 4. Preparation before printing
[0203] Fill the prepared semi-solid material into the syringe device configured by the 3D printer, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com