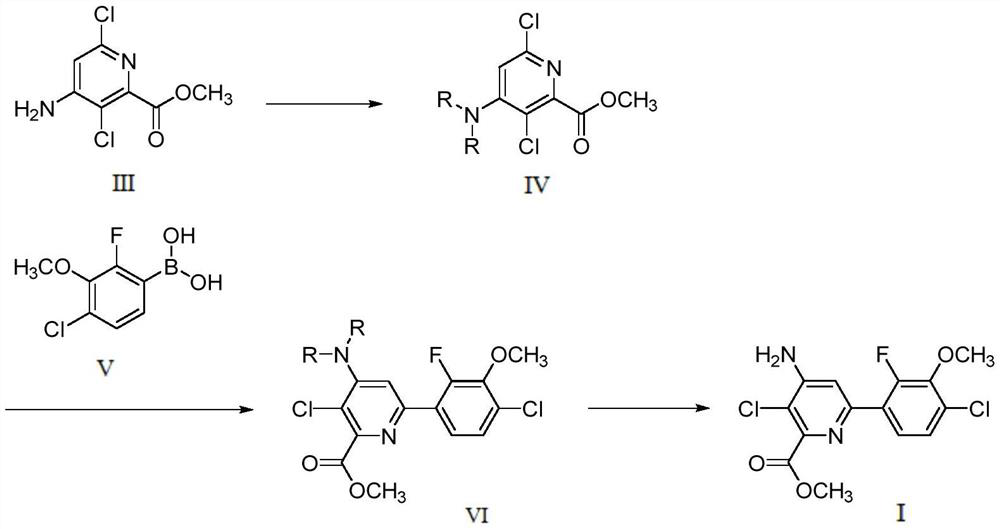
Preparation method of halauxifen-methyl
A technology for fluorochloropyridine ester and dichloropyridine, which is applied in the field of preparation of fluorochloropyridine ester, can solve the problems of unobtainable raw materials, unsuitability for industrial production and the like, and achieves the effects of high reaction efficiency, shortened production cycle and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
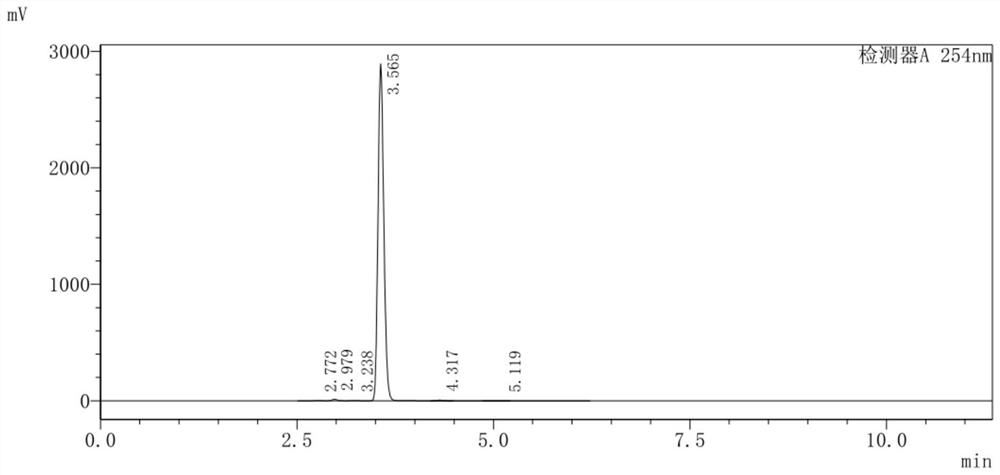
Image
Examples
Embodiment 1
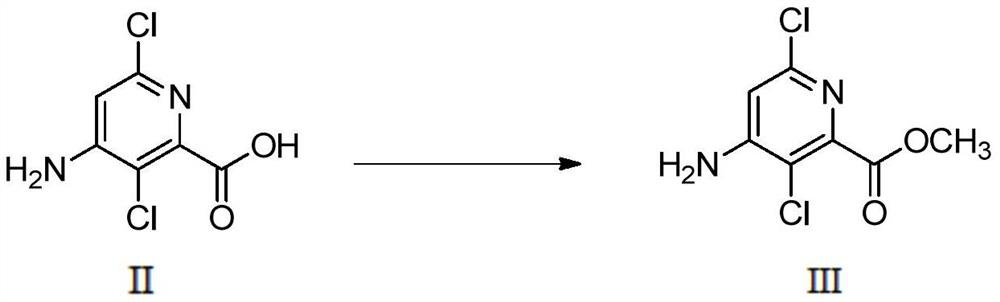
[0039] (1) Preparation of 4-amino-3,6-dichloropyridine-2-carboxylic acid methyl ester (III)
[0040]Add 4-amino-3,6-dichloropyridine-2-carboxylic acid (II) (5.00g, 24.15mmol) into a 100mL eggplant-shaped bottle, add 20mL methanol, stir to dissolve, and drop Thionyl chloride (5.75g, 48.31mmol) was added. After the addition was complete, the reaction was carried out at 60°C. The solution gradually became clear and stirred for 7 hours. The reaction was complete as monitored by TLC. The heating was stopped, cooled to room temperature, and the solvent was evaporated under reduced pressure. Adjust the pH to 9 with 25-28% ammonia water at -5-0°C to precipitate a powdery white solid, filter it with suction, wash with water (30mL×3), and dry to obtain 4.98g of a pink solid, with a yield of 93.26%.
[0041] 1 H-NMR (300MHz, DMSO-d 6 ),δ(ppm):7.10(s,2H,-NH 2 ),6.83(s,1H,-ArH),3.89(s,3H,-CH 3 ).
[0042] (2) Preparation of 4-(N,N-diamido)-3,6-dichloropyridine-2-carboxylic acid methyl...
Embodiment 2
[0052] (1) Preparation of 4-amino-3,6-dichloropyridine-2-carboxylic acid methyl ester (III)
[0053] Add 4-amino-3,6-dichloropyridine-2-carboxylic acid (II) (5.00g, 24.15mmol) into a 100mL eggplant-shaped bottle, add 20mL methanol, stir to dissolve, and drop Add concentrated sulfuric acid (1.54mL), move to react at 60°C after dropping, the solution gradually clarifies, stir and react for about 7 hours, TLC monitors that the reaction is complete, stop heating, cool to room temperature, evaporate the solvent under reduced pressure, and store at -5~0°C The pH was adjusted to 9 with 25-28% ammonia water, and a powdery white solid was precipitated, filtered with suction, washed with water (30mL×3), and dried to obtain 4.76g of a pink solid, with a yield of 89.14%.
[0054] (2) Preparation of 4-(N,N-diamido)-3,6-dichloropyridine-2-carboxylic acid methyl ester (IV)
[0055] Add intermediate III (2.00g, 9.05mmol) into a 25mL eggplant-shaped flask, add 10mL of acetonitrile, stir to di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com