B and N co-doped Co-based high-active oxygen reduction electrocatalyst as well as preparation and application thereof
An electrocatalyst and co-doping technology, applied in circuits, electrical components, battery electrodes, etc., can solve problems such as complex patent process and environmental hazards, and achieve the effects of avoiding application restrictions, improving product performance, and overcoming the decline in catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A method for preparing a Co-based highly active oxygen reduction electrocatalyst co-doped with B and N, specifically comprising the following steps:
[0034] (1) Dissolve 1,3,5-benzenetricarboxylic acid, dicyandiamide, boric acid, and cobalt source in a solvent, and use ultrasonic dissolution to fully react to form a product. The reaction temperature is 50-70°C, and the reaction time is 10-14h, in this step, the cobalt ions in the cobalt source are doped into the carbon support, wherein the cobalt source is CoCl 2 ·6H 2 O, 1,3,5-benzenetricarboxylic acid, dicyandiamide, CoCl 2 ·6H 2 The mass ratio of O and boric acid is 1:10:(0.4~0.6):(0.025~0.075), and the solvent is that ethanol and water are obtained by mixing 1:3.5 by volume;
[0035] (2) Collect the product of step (1) and dry it under vacuum condition, the drying temperature is 50-70°C, and the drying time is 7-10 hours;
[0036] (3) Annealing the obtained dried product under an inert gas, the temperature of t...
Embodiment 1
[0040] A B, N co-doped Co-based highly active oxygen reduction electrocatalyst for fuel cells is obtained by the following preparation steps:
[0041] (1) According to the mass ratio of 1:10:0.5:0.05, take 0.1g of 1,3,5-benzenetricarboxylic acid, 1g of dicyandiamide, 50mg of cobalt chloride hexahydrate, 5mg of boric acid and dissolve in volume ratio of In a mixed solvent of ethanol / water of 1:3.5, ultrasonically dissolved, and reacted at 60° C. for 12 hours to obtain the product.
[0042] (2) The above reaction system was centrifuged to collect the product, and the product was vacuum-dried at 60° C. for 8 hours.
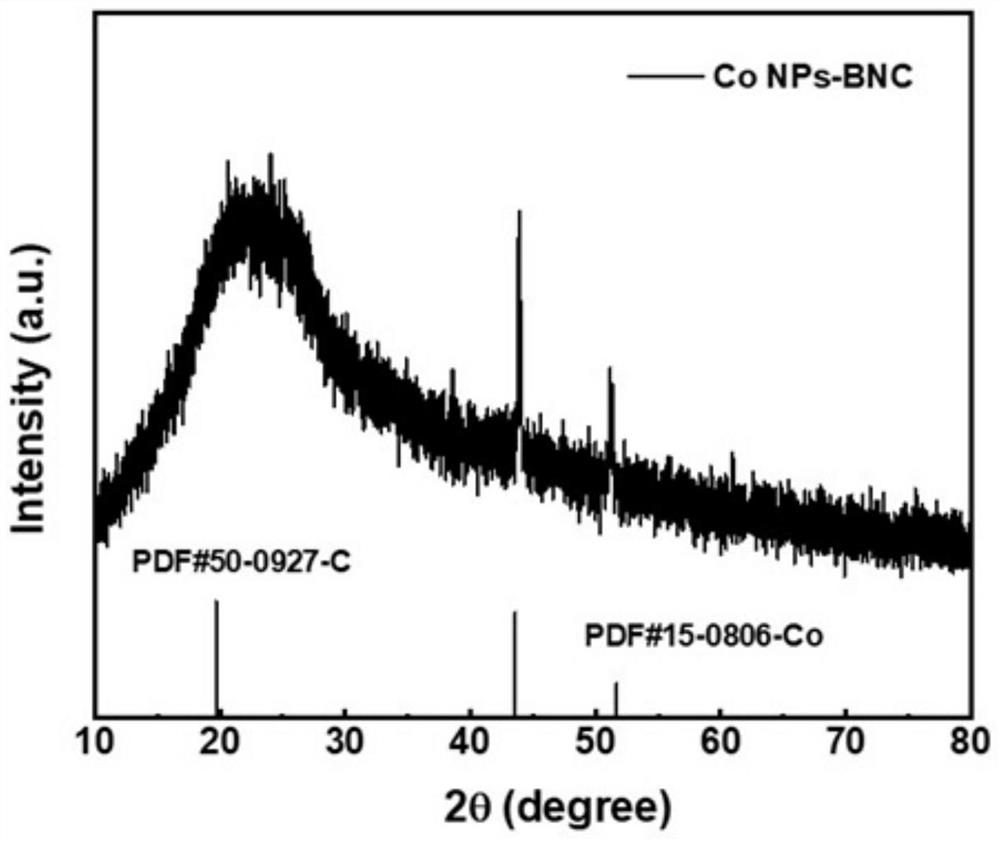
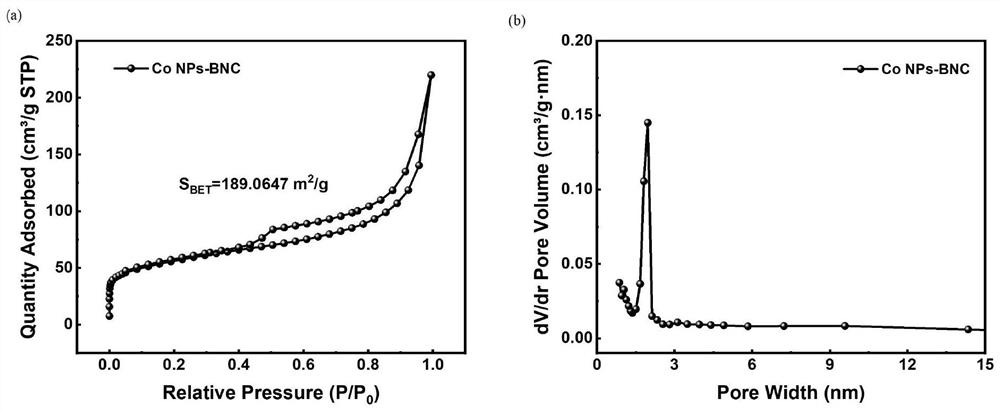
[0043](3) Place the obtained dried product in a porcelain boat and anneal at 900°C for 2 h under an argon atmosphere, and then cool to room temperature to obtain B and N co-doped carbon nanosheets dispersed with Co nanoparticles, denoted as Co NPs-BNC.
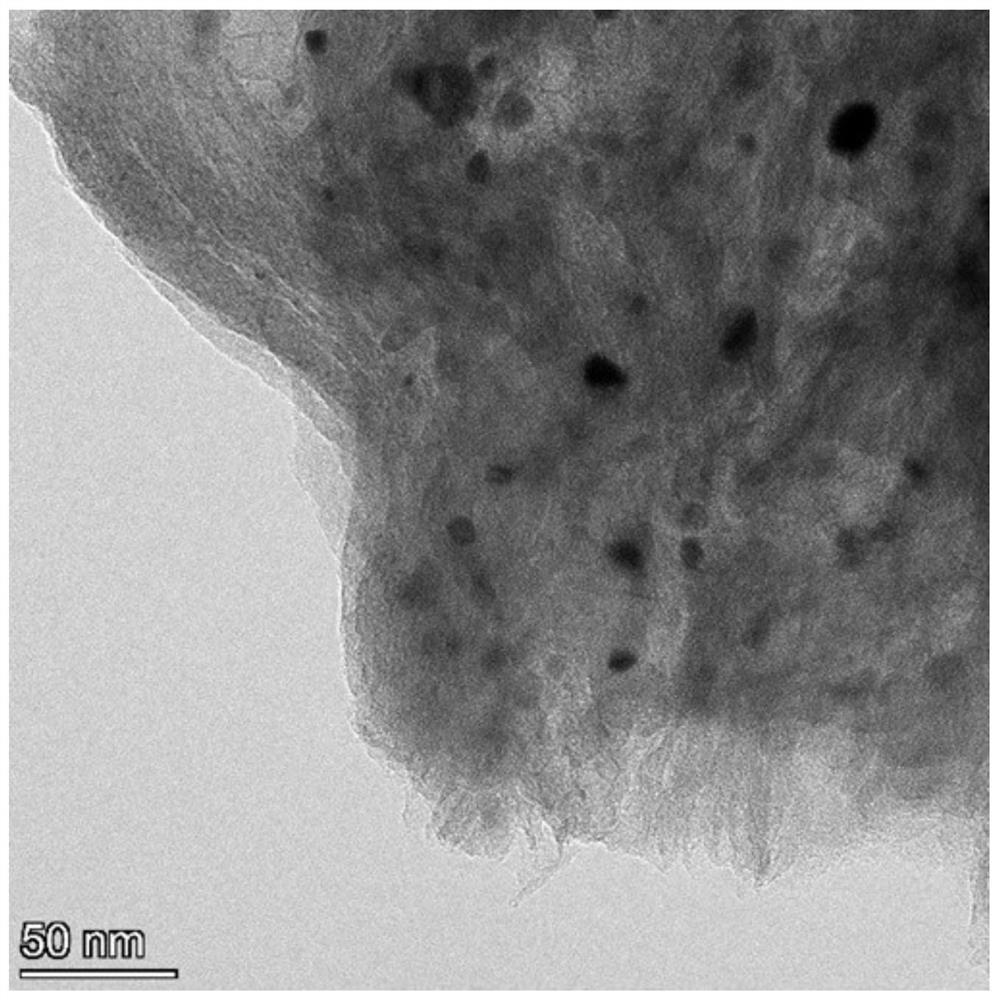
[0044] TEM image of Co NPs-BNC figure 1 As shown, it can be seen that the overall profile of the catalyst is a ...
Embodiment 2
[0058] A B, N co-doped Co-based highly active oxygen reduction electrocatalyst for fuel cells is obtained by the following preparation steps:
[0059] (1) According to the mass ratio of 1:10:0.5:0.025, take 0.1g of 1,3,5-benzenetricarboxylic acid, 1g of dicyandiamide, 50mg of cobalt chloride hexahydrate, 2.5mg of boric acid and dissolve in volume ratio In a mixed solvent of ethanol / water of 1:3.5, ultrasonically dissolved, and reacted at 60° C. for 12 hours to obtain the product.
[0060] (2) The above reaction system was centrifuged to collect the product, and the product was vacuum-dried at 60° C. for 8 hours.
[0061] (3) The obtained dried product was placed in a porcelain boat and annealed at 900° C. for 2 h under an argon atmosphere, and then cooled to room temperature to obtain B and N co-doped carbon nanosheets dispersed with Co nanoparticles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com