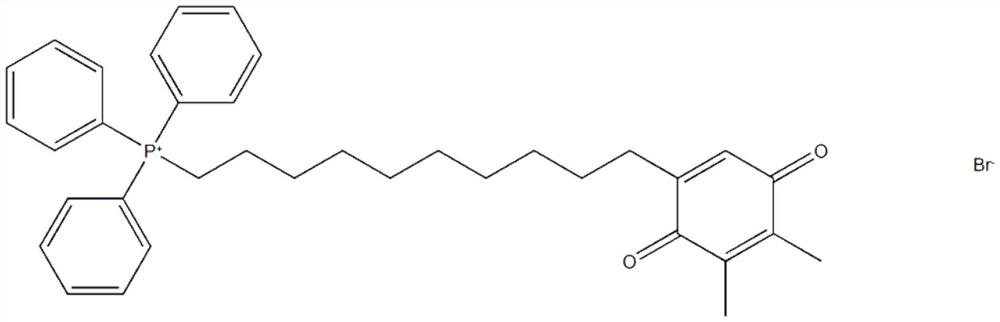
Application of derivative SKQ1 of plastoquinone in preparation of anti-mycobacterium tuberculosis drugs
A technology of mycobacterium tuberculosis and mycobacteria, applied in the field of microbial infectious diseases and pharmacy, can solve the problems of poor patient compliance, long treatment cycle, low treatment success rate, etc., and achieve the effect of small molecular weight and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Bacterial Culture and Compound Storage Solution Preparation
[0015] Cultivation of test strains: Mycobacterium tuberculosis H37Rv (ATCC27294), Mycobacterium bovis M.bovis (ATCC19210) and 6 strains of drug-resistant Mycobacterium tuberculosis isolated from the hospital were inoculated in 7H9 liquid medium and cultured until the Several phases (optical density OD 600 0.8-1.0), the entire operation was carried out in a biosafety cabinet in a biosafety level 3 laboratory (BSL-3).
[0016] Streptococcus suis SC19 (isolated from a pig farm in Sichuan) was inoculated in TSB liquid medium and cultured to logarithmic phase (OD 600 0.8-1.0); Escherichia coli (ATCC25922), Listeria monocytogenes (ATCC19115), Pseudomonas aeruginosa (ATCC9027) and Klebsiella pneumoniae (CTCC46117) were all inoculated in LB liquid medium and cultivated to logarithmic Period (OD 600 is 0.6-0.8).
[0017] Preparation of the mother solution of the compound to be tested: the CSA number of S...
Embodiment 2
[0018] Embodiment 2 determines the minimum inhibitory concentration (MIC) of SKQ1
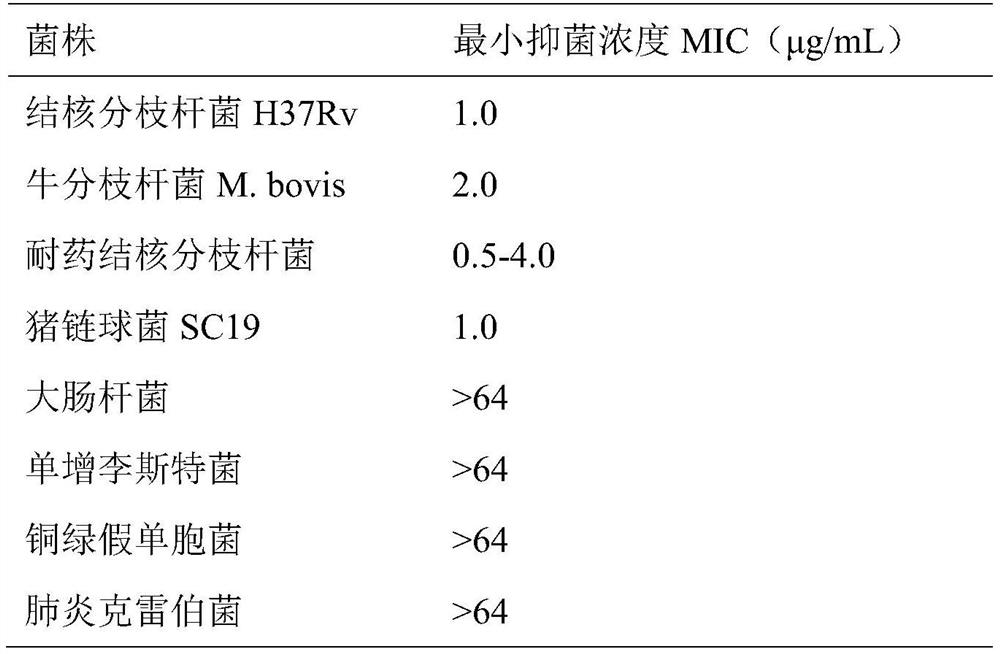
[0019] MIC is defined as the minimum concentration of a compound that inhibits 90% of the growth of bacteria in the medium. Carry out 2-fold serial dilution of the compound to be tested (the dilution gradient is 64 μg / mL-0.125 μg / mL), and the Mycobacterium tuberculosis, Mycobacterium bovis M.bovis, drug-resistant Mycobacterium tuberculosis and Streptococcus suis SC19 were diluted to OD with corresponding liquid medium 600 Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Klebsiella pneumoniae that have grown to the logarithmic phase were diluted to OD with LB liquid medium 600 is 0.001. The diluted bacterial solution was added to the culture medium containing different concentrations of compounds, and a blank control group with only solvent was set, and cultured in a 37°C incubator. By measuring the OD value of each concentration gradient and blank control group, compared ...
Embodiment 3
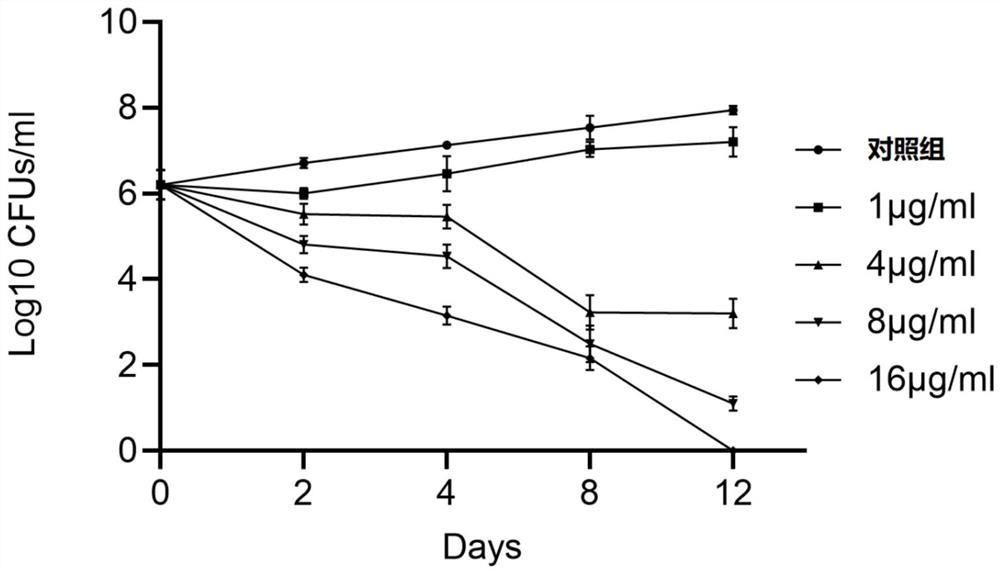
[0023] Dilute the Mycobacterium tuberculosis H37Rv bacteria solution grown to the logarithmic phase with 7H9 liquid medium to OD 600 was 0.01, the stock solution of SKQ1 prepared in Example 1 was added to the diluted bacterial solution respectively to obtain the compound groups whose SKQ1 concentrations were 1.0 μg / mL, 4.0 μg / mL, 8.0 μg / mL and 16.0 μg / mL, In addition, 1% DMSO was set as the control group, cultured in a 37°C incubator for 12 days, and on the 0th, 2nd, 4th, 8th and 12th days of culture, each day was neutralized from the control group. 100 μl was taken out of the compound group with a concentration of 10 times and spread on the 7H11 solid plate medium for counting. The solid plate was cultured in an incubator for 21 days to count the counting results and calculate the colony forming units (CFU). The result is as figure 1 As shown, compared with Control 1, SKQ1 at 4.0 μg / mL was able to kill 99.99% of Mycobacterium tuberculosis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com