Interleukin 2 mutant
A mutant and interleukin technology, applied in the field of protein engineering, can solve problems such as reduced affinity and reduced biological activity of IL-2 mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
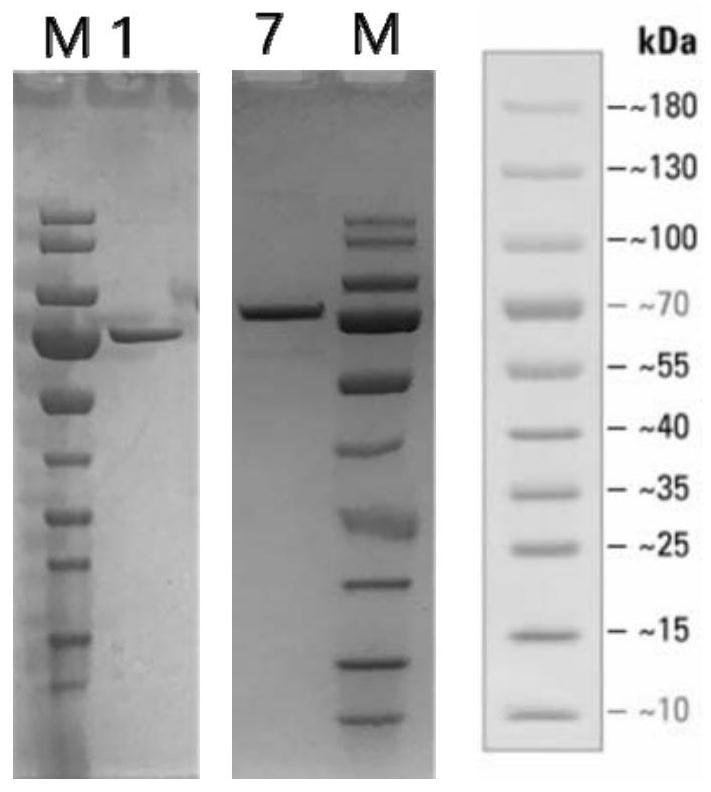
[0118] Example 1. Synthesis of Mutant Interleukin-2 (IL-2) Proteins
[0119] 1. Gene synthesis
[0120] The nucleotide sequence encoding the mutant amino acid sequence of the interleukin-2 (IL-2) protein is obtained by an automatic gene synthesis method. In the example, adding an HSA tag to the end of the gene fragment facilitates purification, and this tag is also a common means to prolong the half-life of protein drugs. The gene fragment is flanked by a single restriction enzyme cleavage site. All gene synthesis sequences were designed with a 5' DNA sequence encoding a leader peptide that targets the protein for secretion in eukaryotic cells.
[0121] Number of mutations mutation site Example mutant names protein tag 3 3, 42 and 44 IL-2gm17 (T3A, F42N, F44T) HSA
[0122] 2. Plasmid construction
[0123] The synthesized gene was subcloned into the pTT5 plasmid using molecular biology reagents according to the manufacturer's instructions.
[...
Embodiment 2
[0126] Example 2. Preparation of receptor protein
[0127] In order to study the binding ability of IL-2 mutant molecules to IL-2Rα receptor and IL-2Rβγ heterodimerization receptor, human IL-2Rα receptor and IL-2Rβγ heterodimerization receptor proteins were prepared.
[0128] The human IL-2Rα receptor was designed by connecting the coding sequence of the extracellular domain of IL-2Rα to the coding sequence of 6×His Tag (SEQ ID NO: 3), and cloning it into the eukaryotic expression vector. 293E cells cultured in Freestyle medium were used for transient transfection expression of IL-2Rα receptor. 24 hours before transfection, inoculate 0.5×10 6 Cells / ml 150ml of 293E cells, 37°C 5% CO 2 Cultivate on a shaker at 120rpm in an incubator. During transfection, add 150 μl of 293fectin to 2.85 ml of OptiMEM, mix well, and incubate at room temperature for 2 minutes; meanwhile, dilute 150 μg of the plasmid used to express IL-2Rα receptor to 3 ml with OptiMEM. Mix the above-mentioned ...
Embodiment 3
[0130] Example 3. Using biacore to detect the affinity experiment of binding receptors
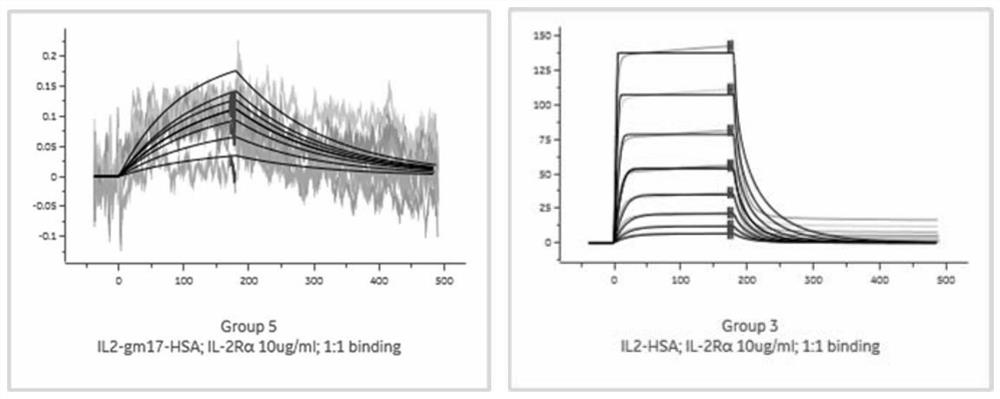
[0131] To study the affinity of IL-2 mutants to the receptor relative to wild type, IL-2 mutant molecules IL-2gm17-HSA and wild-type Affinity of type IL-2-HSA to human IL-2Rα subunit: Human IL-2Rα subunit was immobilized on a CM5 chip (190RU). IL-2gml7-HSA and IL-2-HSA were used as analytes in HBS-EP buffer at 25°C. For IL-2Rα, the analyte concentration was 200 nM down to 1.526 nM (1 :2 dilution) at a flow rate of 30 μl / min (180 sec association time, 300 sec dissociation time). For IL-2Rα, regeneration was performed with 20 mM NaOH, 30 ul / min for 10 seconds. For IL-2Rα, 1:1 binding was used, RI≠0, Rmax=global fit data.
[0132] The result is as figure 2 As shown, the Rmax value of IL-2gm17-HSA was 0, and the Rmax value of IL-2-HSA was 140. IL-2gm17-HSA abolishes the affinity for IL-2Rα relative to IL-2-HSA.
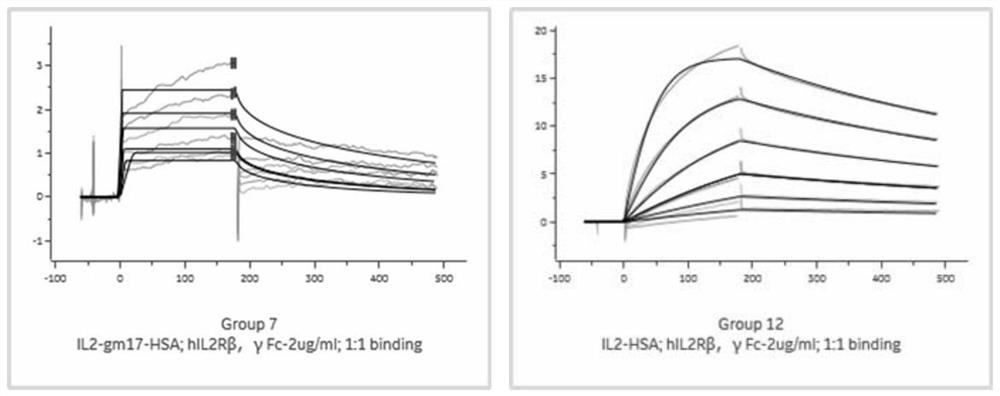
[0133] The affinity of IL-2 mutant molecule IL-2gm17-HSA and wild-type IL-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com