Rapid construction method and application of ACE2 humanized mouse model
A human-derived, transgenic vector technology, applied in biochemical equipment and methods, applications, botanical equipment and methods, etc., can solve the requirements of high experimental conditions, need to go through line establishment, expression verification, etc., the preparation cycle is not less than 9 -12 months time, high failure rate issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
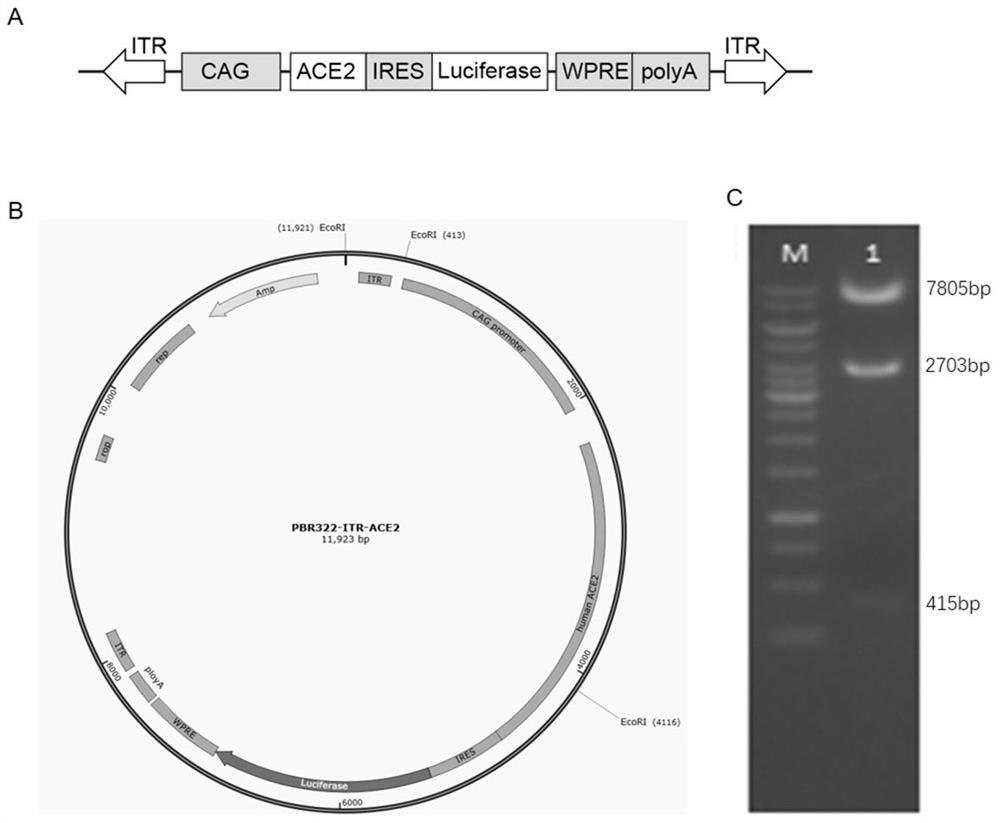
[0015] ACE2 humanized mouse model construction.
[0016] 1) Construction of Piggybac transgene vector: PiggyBac transposon 5' inverted repeat (ITR), CAG promoter, human ACE2 coding region, ribosome access site (IRES), luciferase ), woodchuck hepatitis post-transcriptional regulatory element (WPRE), polyadenylation (polyA) site and 3' inverted repeat (ITR) sequences are SEQ ID NO: 1-8. The CAG promoter was amplified by PCR amplification using the plvct-tTR-KRAB plasmid as a template, and the remaining fragments were obtained by total gene synthesis. During the construction process, the two ITRs and restriction sites were first connected into the PBR322 vector by the In-Fusion method to obtain the PBR322-ITR backbone vector; then the CAG promoter, synthetic human ACE2 fragment, IRES-Luciferase-WPRE -polyA was connected into the PBR322-ITR backbone vector by In-Fusion to obtain the final PBR322-ITR-ACE2 transgenic vector. After the vector was digested and sequenced and verified...
Embodiment 2
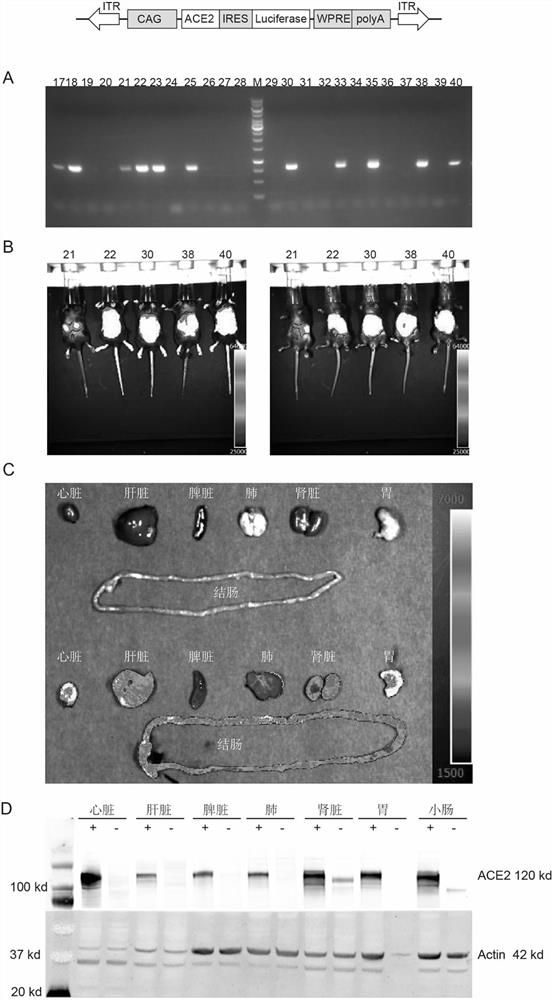
[0024] Construction of ACE2 humanized mouse SARS-CoV-2 virus susceptibility model
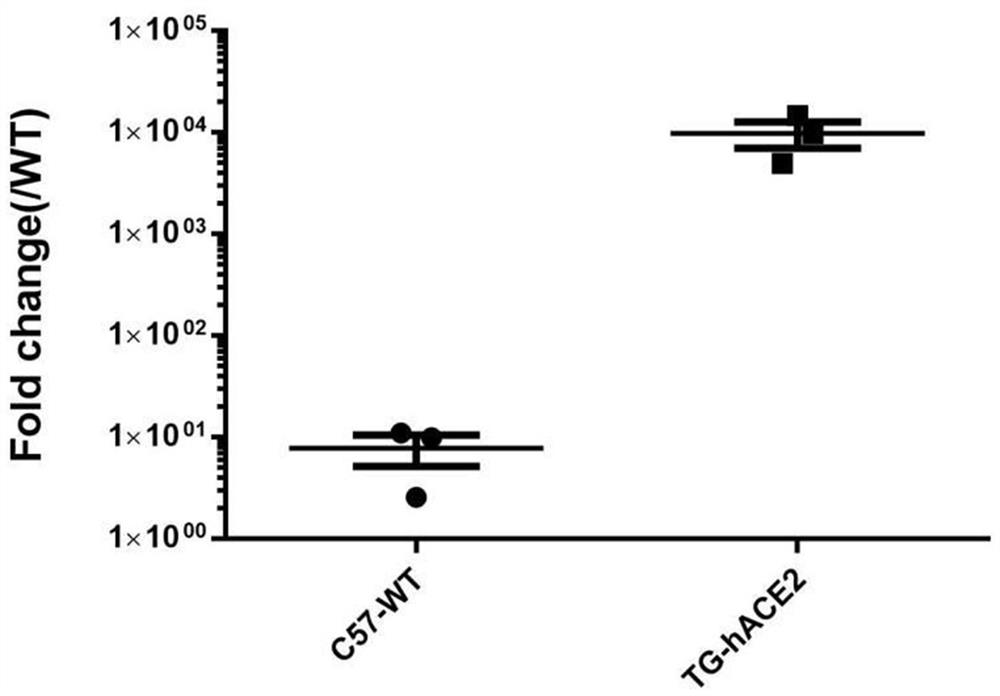
[0025] ACE2 humanized mouse SARS-CoV-2 virus infection and detection experiments were completed in a biosafety level 3 laboratory (BSL-3), and 5 F0 mice with positive expression of Luciferase and 5 wild-type C57BL / 6 In the biological safety cabinet, 50 μL (4.15 × 10 4 PFU) SARS-CoV-2 virus solution was instilled into the nasal cavity of mice. After 4 days of infection, the lung tissues of the mice were collected, RNA was extracted and reverse-transcribed, and real-time fluorescent quantitative PCR (Realtime PCR) was performed to detect the presence of The content of SARS-CoV-2 virus, the results are as follows image 3 As shown, the results show that: 4 days after SARS-CoV-2 virus infection, the virus content in the lung tissue of ACE2 humanized mice positive for Luciferase expression is about 1000 times that of wild-type mice, indicating that ACE2 humanized mice have Novel coronavirus pneum...
Embodiment 3
[0027] Evaluation of SARS-CoV-2 neutralizing antibody protection using ACE2 humanized mice.
[0028] group number of mice Administration (2h after infection) Dose (mg / kg) Route of administration control group 6 PBS - intraperitoneal injection Neutralizing antibody low dose group 5 Neutralizing Antibody-Low dose 5 intraperitoneal injection Medium dose group of neutralizing antibody 5 Neutralizing Antibody-Medium dose 10 intraperitoneal injection Neutralizing antibody high dose group 5 Neutralizing Antibody-High dose 20 intraperitoneal injection
[0029] After infection and administration, the state and body weight of the mice were recorded every day. After 5 days of infection, the lung tissues of the surviving mice were taken, RNA was extracted and reverse-transcribed, and Realtime PCR was performed to detect the presence of SARS-CoV-2 virus in the lung tissues of different groups of mice. content. Experimental r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com