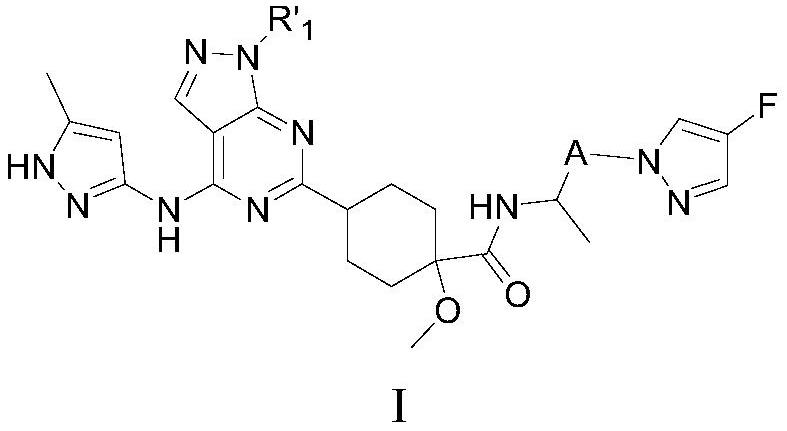
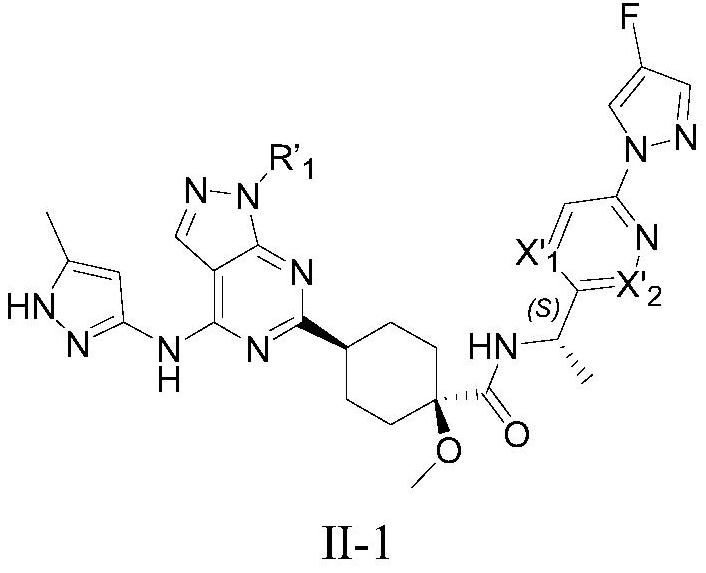
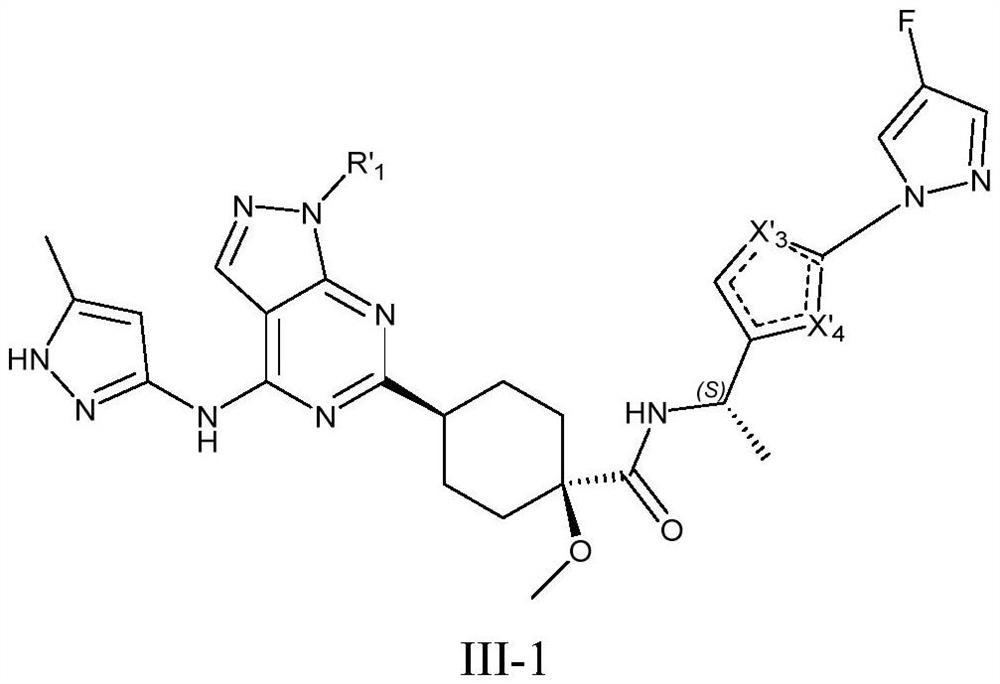
Compound used as RET kinase inhibitor and application thereof
A compound and solvate technology, applied in the field of regulating RET kinase activity or treating RET-related diseases, can solve the problems of VEGFR inhibition toxicity and low targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0404] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula I' or its crystal form, pharmaceutically acceptable salt, hydrate or solvent of the present invention compounds are mixed to form a pharmaceutical composition.
[0405] The present invention also provides a method of treatment, which comprises the steps of: administering the compound of formula I' described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate, to a subject in need of treatment, or Administering the pharmaceutical composition of the present invention for inhibiting RET.
[0406] The present invention has the following main advantages:
[0407] (1) The compound of the present invention has excellent inhibitory ability to RET kinase, especially WT-RET, RET-V804L and RET-V804M;
[0408] (2) The compound of the prese...
Embodiment 1
[0414] The synthetic compound of the present invention:
[0415]
[0416] The experimental process is as follows:
[0417] 1. Synthesis of intermediate C1-7
[0418] The synthetic route is as follows:
[0419]
[0420] 1. Synthesis of C1-9
[0421] C1-8 (6.98g, 34.9mmol), 4-fluoro-1H-pyrazole (3.3g, 35mmol), potassium carbonate (11.1g, 73.6mmol) and DMF (30mL) were added to a 100mL single-necked bottle, at 100°C Reacted for 15 hours, cooled to room temperature, poured into water, filtered and dried to obtain 5.93g of compound C1-9. NMR analysis data of compound C1-9: 1 H NMR (400MHz, CDCl 3 ): δ8.96-8.95(d, J=1.8Hz, 1H), 8.47-8.45(dd, 1H), 8.37-8.34(dd, 1H), 8.05-8.03(dd, 1H), 7.66-7.65(d , J=3.96,1H), 2.65(s,3H).
[0422] 2. Synthesis of C1-10
[0423] Add C1-9 (4.2g, 0.02mol), R-tert-butylsulfinamide (2.48g, 0.02mol), tetraethyl titanate (9.34g, 0.041mol) and THF (50mL) into a 100mL three-necked flask , reacted at 75°C for 15h, cooled to room temperature, pour...
Embodiment 2
[0475] The synthetic compound of the present invention:
[0476]
[0477] The synthetic route and experimental process are as follows:
[0478]
[0479] 1. Synthesis of C2-2
[0480] Compound C2-1 (1.0g, 5.32mmol) was dissolved in dichloromethane (30mL), then DIPEA (2.06g, 15.96mmol) was added, cooled to 0°C in an ice-water bath, and SEM-Cl (2.22g, 13.30 mmol), keep stirring at the temperature for 1.5h, dilute with water, and extract with dichloromethane. The combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography to obtain 1.19 g of compound C2-2. 1 H NMR (400MHz, CDCl 3 )δ8.23(s,1H),5.82(s,2H),3.72-3.68(t,2H),0.99-0.95(t,2H),0.00(s,9H).
[0481] 2. Synthesis of C2-3
[0482] Compound C2-2 (1.09g, 3.43mmol) was dissolved in anhydrous THF (50mL), then potassium tert-butoxide (0.38g, 3.43mmol) and p-methoxybenzyl alcohol ( 0.45g, 3.26mmol) of anhydrous THF (20mL) solution, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com