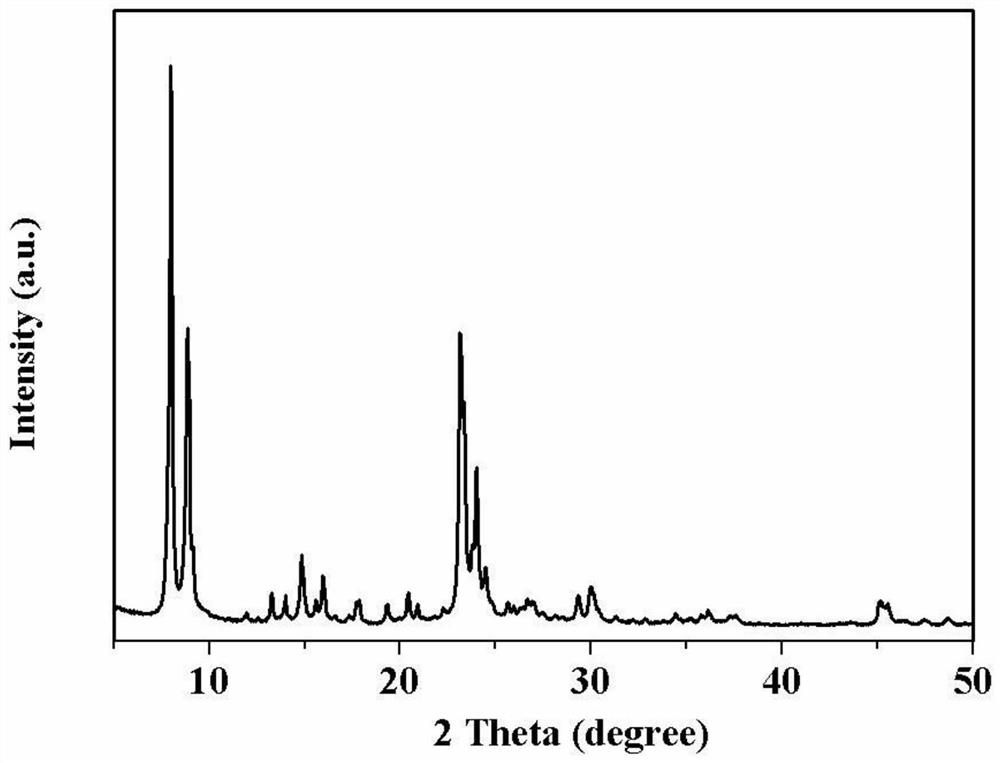
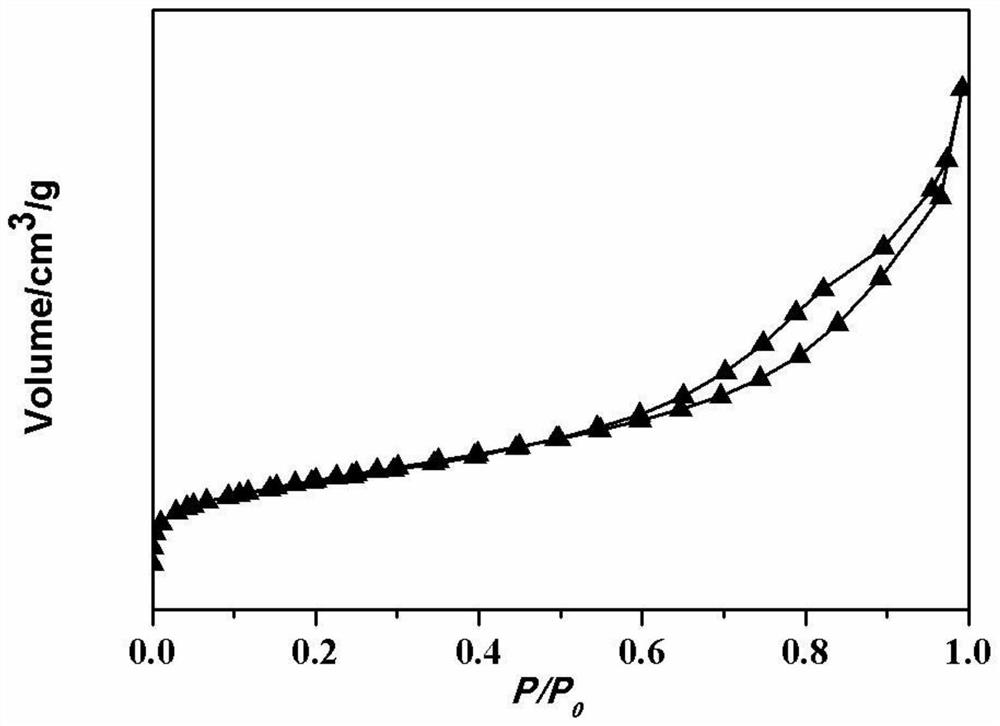
Zn-ZSM-5/ZSM-11 catalyst, preparation method thereof and method for producing aromatic hydrocarbon
A technology of ZSM-11 and catalyst, which is applied in the field of Zn-ZSM-5/ZSM-11 catalyst and its preparation and production of aromatics, which can solve the problem of limited catalytic performance of the catalyst, harsh conditions of aromatization reaction, and easy carbon deposition and deactivation of the catalyst To achieve high liquid yield and aromatics selectivity, solve the harsh conditions of aromatization reaction, improve liquid yield and aromatics selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Preparation of Zn-ZSM-5 / ZSM-11 catalyst (cat1)
[0045] Add 100g of silica sol and 12ml of 4mol / L sodium hydroxide solution into 200g of deionized water, and stir at 25°C for 2h to obtain the mixed solution A1;
[0046] Add 5ml of 1.2mol / L aluminum sulfate solution, 1.54g of tetrabutylammonium hydroxide and 2.75g of zinc nitrate to the above mixed solution A1, stir at 25°C for 2h, then reflux at 90°C for 4h to obtain mixed solution B1 (SiO 2 : Na 2 O: Al 2 o 3 : Templating agent: Zn: H 2 The molar ratio of O is about 83.3:4.0:1.0:0.98:1.54:1851);
[0047] The mixed solution B1 was transferred to a crystallization kettle lined with polytetrafluoroethylene, and hydrothermally crystallized at 150°C for 36 hours to obtain the mixed solution C1;
[0048] Cool the mixed solution C1 to room temperature, filter, wash to pH = 8-9, and dry at 100°C for 6h. Roast at 550°C for 4 hours to prepare Zn-NaZSM-5 / ZSM-11 molecular sieve;
[0049] Soak Zn-NaZSM-5 / ZSM-11 molecular ...
Embodiment 2
[0056] 1. Preparation of Zn-ZSM-5 / ZSM-11 catalyst (cat2)
[0057] Add 210g of silica sol and 15ml of 4mol / L sodium hydroxide solution into 180g of deionized water, and stir at 25°C for 3h to obtain the mixed solution A2;
[0058] Add 5ml of 1.2mol / L aluminum sulfate solution, 2.86g of tetrabutylammonium hydroxide and 2.75g of zinc nitrate to the above mixed solution A2, stir at 30°C for 3.5h, then reflux at 90°C for 4h to obtain mixed solution B2 (SiO 2 : Na 2 O: Al 2 o 3 : Templating agent: Zn: H 2 The molar ratio of O is about 175.0:5.0:1.0:1.84:1.54:1666.7);
[0059] Transfer the mixed solution B2 to a crystallization kettle lined with polytetrafluoroethylene, and conduct hydrothermal crystallization at 150°C for 36 hours to obtain the mixed solution C2;
[0060]Cool the mixed solution C2 to room temperature, filter, wash to pH = 8-9, and dry at 100°C for 6h. Roast at 550°C for 4 hours to prepare Zn-NaZSM-5 / ZSM-11 molecular sieve;
[0061] After steps such as ammoniu...
Embodiment 3
[0066] 1. Preparation of Zn-ZSM-5 / ZSM-11 catalyst (cat3)
[0067] Add 48g of silica sol and 9ml of 4mol / L sodium hydroxide solution into 200g of deionized water, and stir at 25°C for 3h to obtain mixed solution A3;
[0068] Add 5ml of 1.2mol / L aluminum sulfate solution, 1.47g of tetrabutylammonium hydroxide and 2.75g of zinc nitrate to the above mixed solution A3, stir at 45°C for 3.5h, then reflux at 100°C for 5h to obtain mixed solution B3 (SiO 2 : Na 2 O: Al 2 o 3 : Templating agent: Zn: H 2 The molar ratio of O is about 40.0:3.0:1.0:0.94:1.54:1851);
[0069] Transfer the mixed solution B3 to a crystallization kettle lined with polytetrafluoroethylene, and conduct hydrothermal crystallization at 150°C for 36 hours to obtain the mixed solution C3;
[0070] Cool the mixed solution C3 to room temperature, filter, wash to pH = 8-9, and dry at 100°C for 6h. Roast at 550°C for 4 hours to prepare Zn-NaZSM-5 / ZSM-11 molecular sieve;
[0071] After steps such as ammonium excha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com