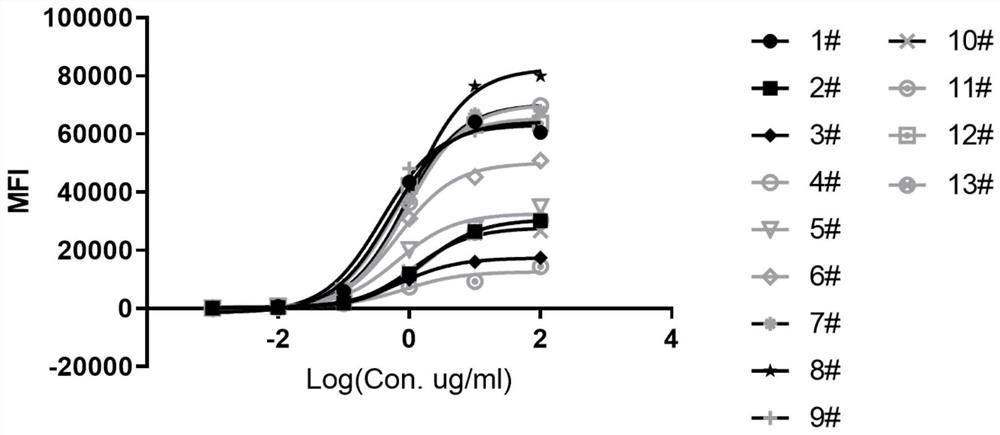
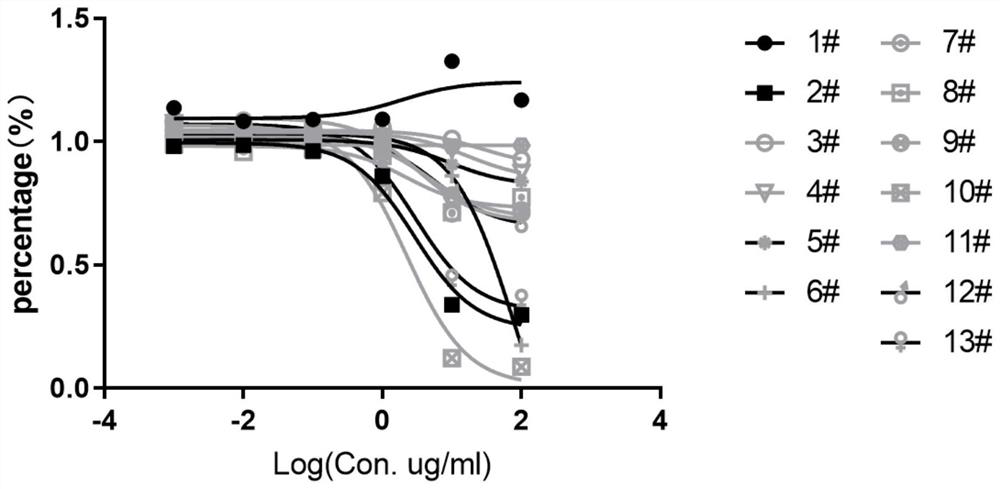
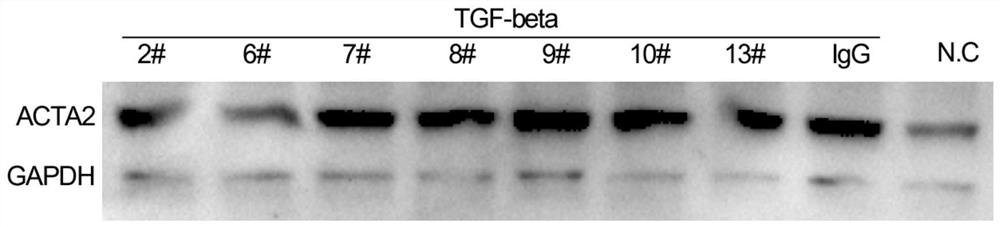
Anti-IL-11R antibody and application thereof
A technology of antibody and variable region, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, etc., can solve the problem of unsatisfactory curative effect and achieve Good clinical application prospects, inhibition of fibrosis, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation of embodiment 1 antigen
[0117] Design a fusion protein of signal peptide, human IL-11RA protein extracellular domain and human IgG1 Fc from the amino terminal to the carboxyl terminal, wherein the amino acid sequence of the signal peptide is SEQ ID NO: 27 1-21 , the amino acid sequence of the extracellular domain of human IL-11RA protein is 22-367 of SEQ ID NO:27. The nucleic acid sequence encoding the fusion protein was constructed on the pcDNA3.4 vector to obtain the expression vector pcDNA3.4-IL11R-Fc; using the transient method (ExpiFectamineCHO Transfection Kit, Thermo Fisher; product number: A29129), the pcDNA3.4-IL11R-Fc was transformed into IL11R-Fc was transfected into mammalian cell CHO and expressed for 14 days; the cell supernatant was harvested after the expression was completed, and purified using Protein A purification column (GE Healthcare, Cat: 17040201); after ultrafiltration, the recombinant protein used for immunizing animals was ob...
Embodiment 2
[0118] Embodiment 2 animal immunization
[0119] Female Balb / c mice (purchased from Hunan Slack Jingda Experimental Animal Co., Ltd.) were taken, and the recombinant protein antigen IL-11R-Fc prepared in Example 1 was fully emulsified with an immune adjuvant to immunize the animals. The immunization process is as follows: Table 4 shows.
[0120] Table 4 Arrangement of immunized animals
[0121]
[0122]
Embodiment 3
[0124] Example 3 Fusion and screening of antibody-secreting cells
[0125] The mouse splenocytes isolated in Example 2 were fused with the immortalized mouse myeloma cells SP2 / 0, and the obtained fused cells were plated in a 96-well plate for pressurized selection and cultured statically for about 14 days. Wherein the medium in the 96-well plate is configured according to the following formula: 1 × HAT medium supplement (Gibco, H0137-10VL), 10% Hybridoma Feeder addition factor (Boaolong, CM-2001), 1 × L-Glutamine 200mM ( RPMI 1640 medium of Gibco, 25030-081), 1 x Penicillin-Streptomycin (Gibco, 15140-122) and 10% FBS (Gibco, 10091-148). After the fused cells grew into clonal clusters, the cell supernatant was taken for antigen-binding ELISA screening. Through ILISA screening, the fused cell mass that can bind to IL-11R-his was transferred to a 24-well plate and cultured for 3 days, and then the supernatant was taken for ELISA affinity detection again. The cells that were bou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com