Drug gene mutation detection kit and detection method based on SNV array technology
A kit and drug technology, applied in the field of drug gene mutation detection kits based on SNVarray technology, can solve problems such as false positives and false negatives, inability to provide clinical guidance for patients with medication, and difficulty in ensuring detection accuracy, achieving high convenience and comprehensiveness. Pharmacogene mutation detection and the effect of improving the degree of standardization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
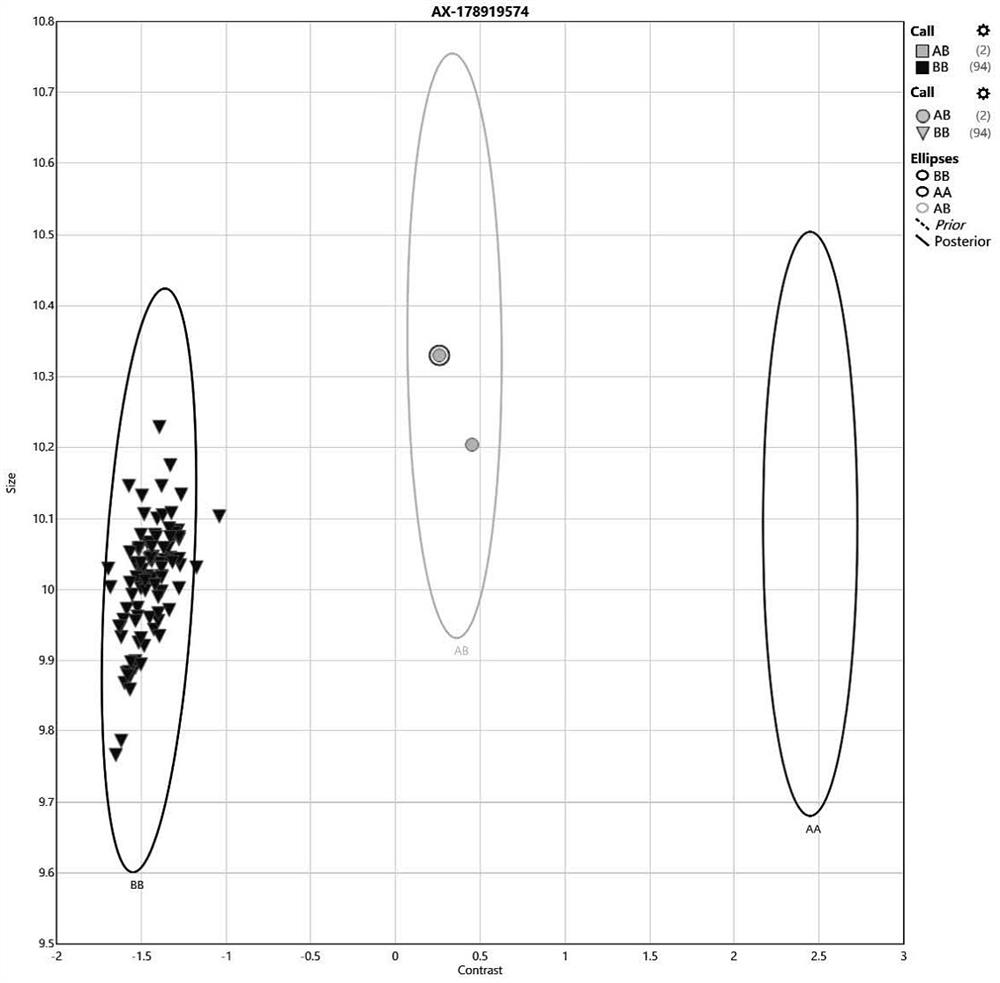
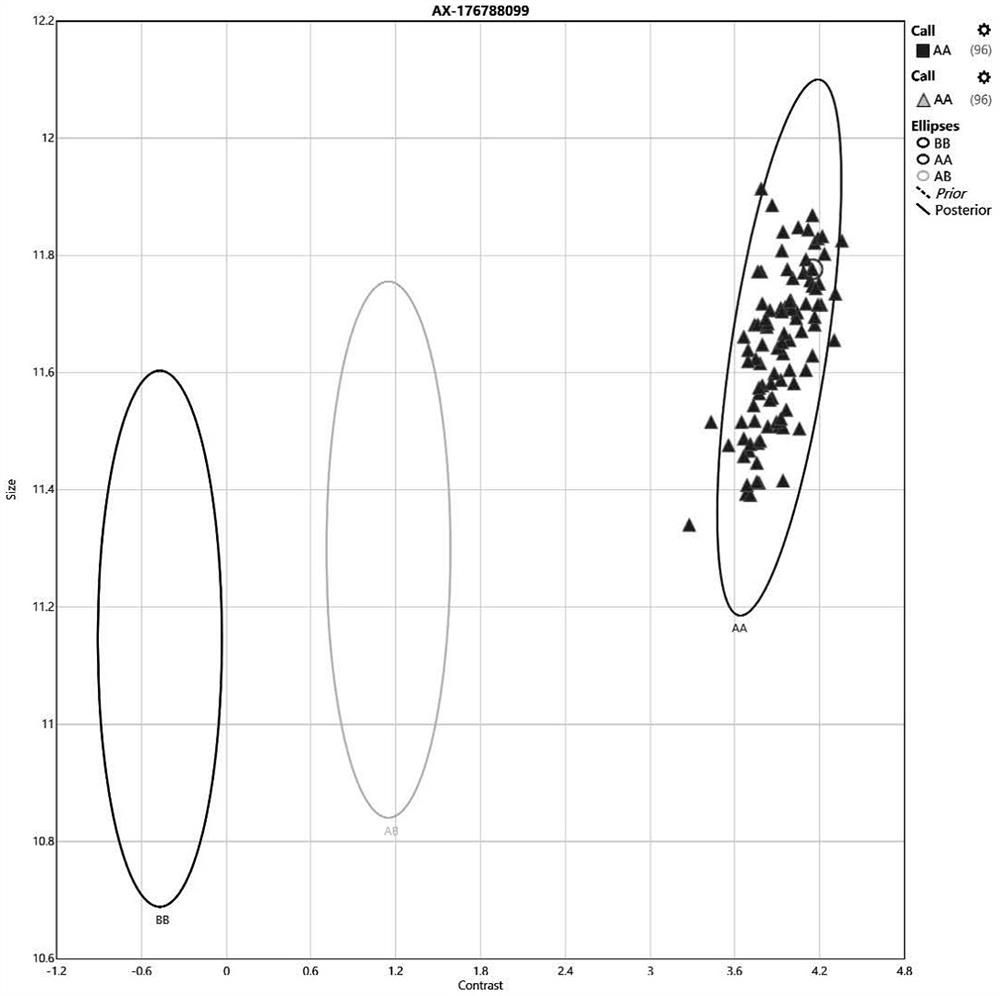
[0056] A drug gene mutation detection kit based on SNV array technology in this embodiment, the kit includes the probe set shown in SEQ ID No.1 to SEQ ID No.63.
[0057] In the above kit, preferably, the design of the probe set is combined with the literature reports of clinical laboratories, aiming at the clinical diagnosis and treatment guidelines related to pharmacogenes, and combining the databases such as ClinVar, CPIC and pharmaGKB to carry out population frequency analysis of these gene-related loci. Statistics and screening have confirmed the high-incidence sites of the Chinese population's genetic characteristics for each gene, and synthesized corresponding probes for these sites and made them into chips, with at least one probe for each site. Some sites are 2 repeated probes. This is to place the same probes at different positions on the chip, thereby increasing the accuracy of the results and the positive detection rate of the corresponding gene sites. The screening ...
Embodiment 2
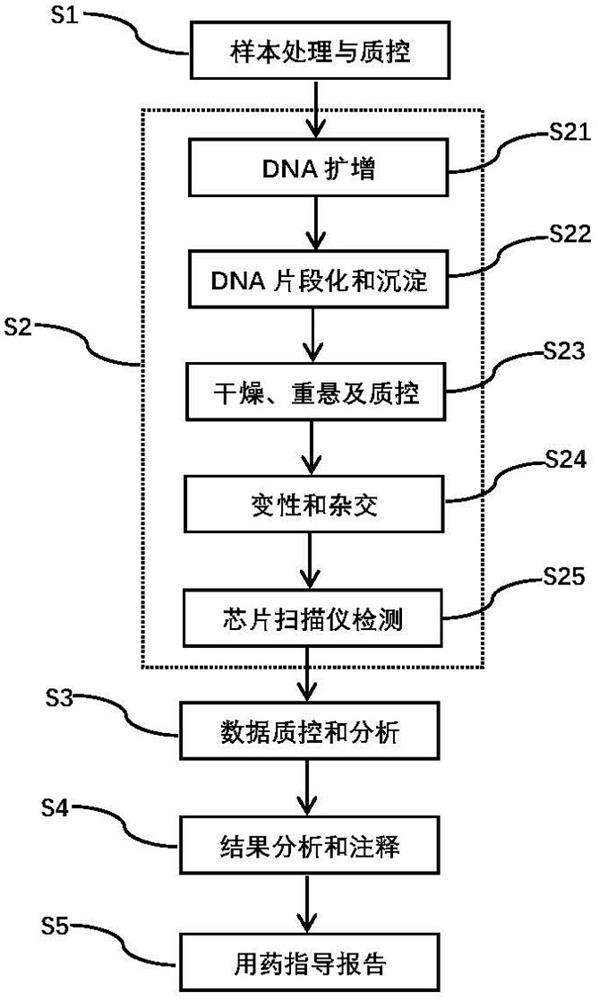
[0078] A kind of drug gene mutation detection method based on SNV array technology in the present embodiment comprises the following steps:
[0079] S1: Sample processing and quality control: 12 clinically obtained samples were used as positive verification samples for the invention. These verification samples are first subjected to DNA extraction, and quality control is carried out through gel electrophoresis, Nanodrop, etc. to ensure that each DNA sample has no degradation, no impurity contamination, and high purity. The optical density (Optical Density, OD) 260 / 280nm ratio is between 1.8 and 2.0, and the OD 260 / 230nm ratio is between 1.5 and 2.0. Samples that do not meet any of the conditions need to be purified. The gel loading dye is Invitrogen TM TrackIt Cyan / Orange Loading Buffer (Invitrogen P / N 10482-028); 25bpInvitrogen Ladder (Invitrogen P / N 10488-022); Gel Electrophoresis System is Invitrogen E-Gel TM 48 agarose gels 4%, G8008-04.
[0080] S2: detection response...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com