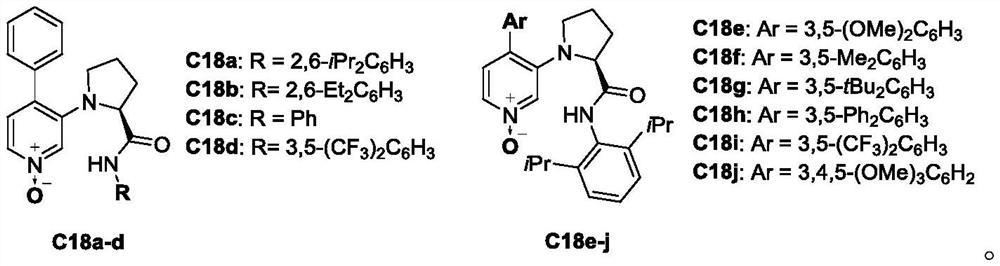
Chiral 3-amino-4-arylpyridine nitrogen-oxygen catalyst and application thereof in tetrazole hemiacetal amine ester reaction
A technology of arylpyridine nitrogen oxide catalyst and aryl pyridine nitrogen oxide, which is applied in the synthesis of chiral 3-amino-4-arylpyridine nitrogen oxide catalyst to catalyze the synthesis of tetrazole hemiaminal ester, a new type In the field of chiral DMAP catalysts, it achieves the effects of strong adjustability, simple synthesis and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of chiral 4-phenyl-3-(2-(2,6 diisopropylbenzamido) pyrrolidinyl) pyridine nitrogen oxide
[0034]
[0035] Under nitrogen protection, chiral 3-pyrrolidinyl-4-chloropyridine nitrogen oxide (125mg, 0.31mmol), phenylboronic acid (152mg, 1.24mmol), potassium carbonate (171mg, 1.24mmol) were sequentially added into a 25mL sealed tube ), Pd(PPh 3 ) 4 (17.90mg, 0.015mmol), Xant-Phos (14.4mg, 0.031mmol) and 2.5mL of toluene were placed in a 120°C oil bath, and the stirring was stopped after 40min of reaction. Monitored by TLC, after the reaction of the raw materials was completed, the crude product was concentrated in vacuum to obtain a crude product, and then separated by column chromatography to obtain a light brown solid. The raw material chiral 3-pyrrolidinyl-4-chloropyridine nitrogen oxide in this example can be synthesized by referring to Angew.Chem.Int.Ed.2019, 58, 2839. 1 H NMR (400MHz, CDCl 3 )δ8.35(s,1H),8.08(s,1H),7.85(d,J=6.4Hz,1H),7.46-7....
Embodiment 2
[0036] Example 2 Chiral 4-phenyl-3-(2-(3,5-bistrifluoromethylbenzamido)pyrrolidinyl)pyridine nitrogen oxide
[0037]
[0038] Under nitrogen protection, chiral 3-pyrrolidinyl-4-chloropyridine nitrogen oxide (125 mg, 0.27 mmol), phenylboronic acid (134 mg, 1.10 mmol), potassium carbonate (1.10 mmol), Pd(PPh 3 ) 4 , (15.59mg, 0.0135mmol), Xant-Phos (17.4mg, 0.027mmol) and 2.5mL of toluene were placed in a 120°C oil bath, and the stirring was stopped after 40min of reaction. Monitored by TLC, after the reaction of the raw materials was completed, the crude product was concentrated in vacuum to obtain a crude product, and then separated by column chromatography to obtain a light brown solid. The raw material chiral 3-pyrrolidinyl-4-chloropyridine nitrogen oxide in this example can be synthesized by referring to Angew.Chem.Int.Ed.2019, 58, 2839. 1 H NMR (400MHz, CDCl 3 )δ10.98(s,1H),8.64(s,1H),7.85(d,J=6.4Hz,1H),7.61-7.47(m,6H),7.46-7.41(m,1H),7.31(s ,1H),7.18(d,J=6.4Hz,1H)...
Embodiment 3
[0039] Example 3 Chiral 4-(3,5-dimethylphenyl)-3-(2-(2,6 diisopropylbenzamido)pyrrolidinyl)pyridine nitrogen oxide
[0040]
[0041] Under nitrogen protection, chiral 3-pyrrolidinyl-4-chloropyridine nitrogen oxide (125 mg, 0.31 mmol), 3,5-dimethylphenylboronic acid (186 mg, 1.24 mmol), and Potassium carbonate (171mg, 1.24mmol), Pd(PPh 3 ) 4 (17.90mg, 0.015mmol), Xant-Phos (14.44mg, 0.031mmol) and 2.5mL of toluene were placed in a 120°C oil bath, and the stirring was stopped after 40min of reaction. Monitored by TLC, after the reaction of the raw materials was completed, the crude product was concentrated in vacuum to obtain a crude product, and then separated by column chromatography to obtain a light brown solid. 1 H NMR (400MHz, CDCl 3 )δ8.20(s,1H),7.83(dd,J=6.4,1.6Hz,1H),7.72(s,1H),7.23(d,J=8.0Hz,1H),7.11(d,J=8.0 Hz, 2H), 7.02(d, J=6.8Hz, 1H), 6.98(s, 1H), 6.95(s, 2H), 4.20(t, J=7.2Hz, 1H), 3.25(dt, J=9.6 ,7.6Hz,1H),2.93-2.75(m,3H),2.48-2.34(m,1H),2.27(s,6H),2.14-2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com