Preparation method and applications of a class of electrophilic enol salts
An enolate, electrophilic technology, applied in the field of metal organic catalysis, can solve the problems of instability, harsh reaction conditions, poor reaction selectivity, etc., and achieve the effects of diverse structures, high tolerance, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
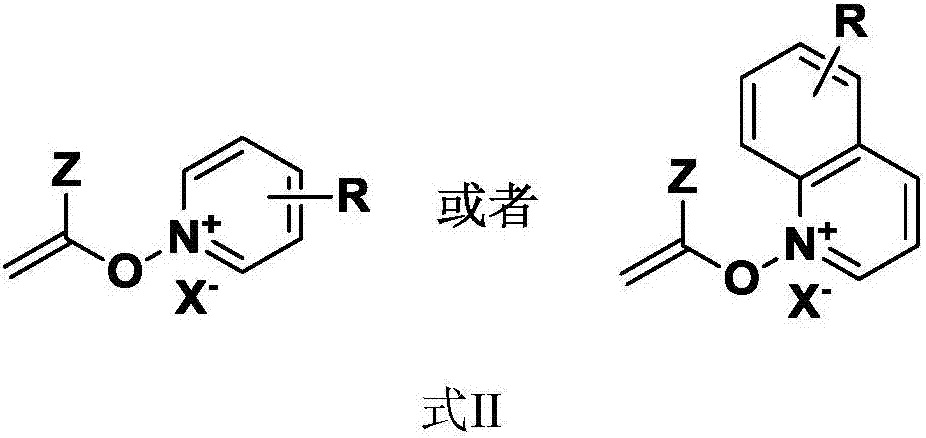
[0030] One aspect of the embodiments of the present invention provides an effective synthesis method of an electrophilic polar enolate, comprising: using a monovalent silver salt as a catalyst to make terminal alkynyl compounds, pyridine or quinoline nitrogen oxides, or derivatives thereof Compounds and proton donors undergo addition reactions in organic solvents to obtain electrophilic enolates.
[0031] Various types of terminal alkynyl compounds, such as terminal alkynes of different structures and containing different functional groups, are applicable to the electrophilic enolate synthesis method of the present invention and have wide applicability.
[0032] Various types of nitrogen oxides, such as pyridine, substituted pyridine nitrogen oxides, quinoline nitrogen oxides, etc., are suitable for the electrophilic enolate synthesis method of the present invention.
[0033] Various types of proton donors, such as HNTf 2 , MsOH, etc. are applicable to the enolate synthesis m...
Embodiment 1
[0041]
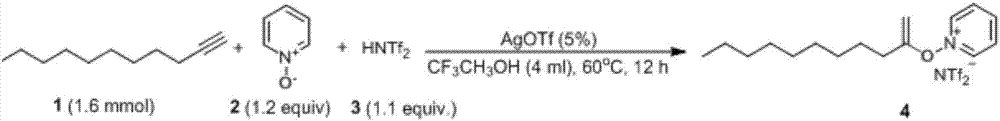
[0042] Prefabricated compound A is a mixture of pyridine nitrogen oxide and acid. Under ice bath conditions, add pyridine nitrogen oxide (1.2equiv 0.456g), Tf 2 NH (1.1 equiv 1.232g), stirred at room temperature for 30 minutes, dichloromethane was distilled off under reduced pressure to obtain light yellow oil.
[0043] Add prefabricated compound A (1.1equiv., 0.828g), 1-undecyne (2mmol0.314g), silver trifluoromethanesulfonate (5%, 35.7mg), trifluoroethanol (4ml ), heated to 60° C., stirred and reacted for 12 hours, TLC monitoring was completed, and flash column chromatography (eluent dichloromethane:methanol=50:1) gave a reddish-brown oily liquid with a yield of 78%.
[0044] The characterization data of product 4 are as follows: 1 H NMR (CDCl 3 , 400MHz): δ8.86(dd, J=6.9,0.9Hz, 2H), 8.66(td, J=7.9,1.2Hz, 1H), 8.29(t, J=7.3Hz, 2H), 4.51(d, J=5.5Hz, 1H), 3.69(d, J=5.5Hz, 1H), 2.38(t, J=7.5Hz, 2H), 1.76-1.50(m, 2H), 1.26(m, 12H), 0.87( t,J=6.9Hz,3H); 13 C NMR (...
Embodiment 2
[0046]
[0047] In a 10ml reaction flask, add pyridine nitrogen oxide (1.2equiv, 0.261g), Tf 2 NH (1.1equiv, 0.616g), 1-undecyne (2mmol, 0.314g), silver acetate (5%, 33.4mg), trifluoroethanol 4ml, heated and stirred at 60°C for 12 hours, TLC monitoring was completed, and the flash column Chromatography (eluent dichloromethane: methanol = 50:1) gave the product as a reddish-brown oily liquid with a yield of 84%.
[0048] The characterization data of the product are as follows: 1 H NMR (CDCl 3 , 400MHz): δ8.86(dd, J=6.9,0.9Hz, 2H), 8.29(t, J=7.3Hz, 2H), 4.51(d, J=5.5Hz, 1H), 3.69(d, J= 5.5Hz, 1H), 3.31(s, 3H), 2.38(t, J=7.5Hz, 2H), 1.76-1.50(m, 2H), 1.26(m, 12H), 0.87(t, J=6.9Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ166.22, 146.70, 141.98, 130.31, 88.93, 67.04, 31.76, 30.90, 29.33, 29.17, 29.09, 28.82, 26.42, 22.58, 14.01; 19 F NMR (CDCl 3 ,376MHz) δ-78.64; F 19 NMR (CDCl 3 ,376MHz)δ-78.78.IR(cm -1 ):2947,2846,1667,1482,1350,1185,1135,1055.ESI + calculated for [C 16 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com