Preparation method of hepatitis B antibody fragment, hepatitis B antibody fragment, kit and application
An antibody fragment, hepatitis B technology, applied in the preparation of hepatitis B antibody fragments, hepatitis B antibody fragments, kits and application fields, can solve the problems of side reactions, non-specific binding false positive diagnosis and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the hepatitis B antibody fragment provided by the application comprises the following steps:
[0035] Add pepsin, dithioerythyl sugar alcohol and 2-mercaptoethanol to the hepatitis B antibody solution, and incubate for the first time; then add glycine, and perform purification after the second incubation. By using pepsin to digest hepatitis B antibody to improve the affinity of the antibody and reduce non-specific binding.
Embodiment 1
[0036] Example 1 Preparation of Hepatitis B Antibody Fragments
[0037] In this embodiment, the following process parameters are taken as examples for illustration.
[0038] 1. Dilute pepsin (Beijing Suolaibao, product number: P8160, 10000U / mg) with citric acid buffer (0.06mol / L pH4.8) to 1mg / ml, adjust the pH value to 2.0 with concentrated hydrochloric acid, and set aside.
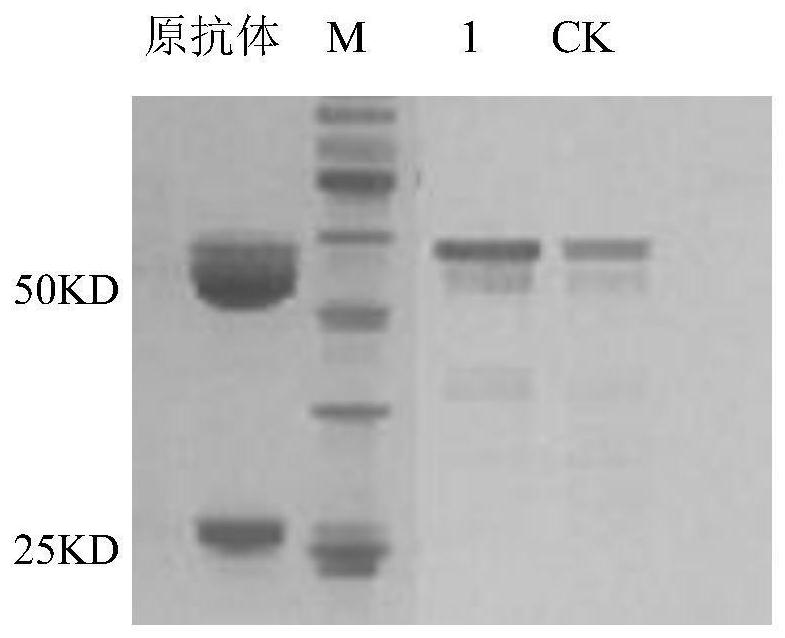
[0039] 2. Take 2ml of hepatitis B antibody (Fei Peng Bio, batch number: 20200518, 5mg / ml) into a 50KD dialysis bag, and dialyze with 100ml of citric acid buffer (0.06mol / L pH4.8) for 30min. After dialysis, cut 1ug of hepatitis B antibody with 1U pepsin, add 0.5ml of the above pepsin dropwise, then add 0.005ml of 5mM dithioerythyl sugar alcohol DTE (1.5g / L) and 0.1ml of 100mM 2-mercaptoethanol (0.3g / L), put the mixture in an oven at 25°C, incubate for 20min, then add 75ul glycine (3.3g / L) with a concentration of 1.5mol / L, and rotate at room temperature for 10min to react. After the reaction, the pH value...
Embodiment 2
[0043] Example 2 Detection of hepatitis B antigen
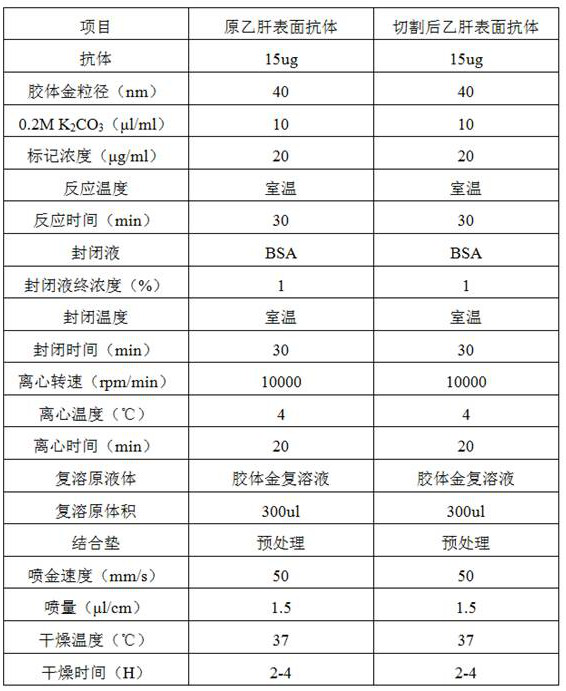
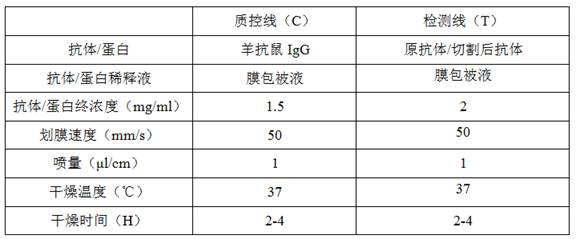
[0044] The hepatitis B antigen was detected by using the hepatitis B antibody fragment (fragment for short) in Example 1. There are many methods for detecting antigens by using antibodies, and colloidal gold testing is used as an example for illustration in this embodiment.
[0045] Membrane coating solution: Tris 1.21g, sucrose 0.5g, EDTA-2Na 0.5g, SDS 0.15g, hydrochloric acid 200ul, dilute to 100ml water.
[0046] Colloidal gold complex solution: Tris 1.21g, sucrose 2.5g, polyvinylpyrrolidone (PVA) 0.05g, BSA 0.5g, polyethylene glycol 0.05g, sodium caseinate 0.5g, hydrochloric acid 200ul, Tween 20 0.5ml , Dilute to 100ml of water.
[0047] Conjugate pad treatment solution: KH 2 PO 4 0.02g, TW20 0.1ml, Na 2 HPO 4 0.29g, 10g sucrose, 1g BSA, 0.05ml PC, dilute to 100ml water.
[0048] Sample pad treatment solution: KH 2 PO 4 0.02g, Na 2 HPO 4 12H 2 O 0.29g, KCl 0.02g, PC 0.05ml, TW201ml, Trition100 0.1ml, dilute t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com