Method for efficiently converting sesquiterpene isomer derivative and preparing high-purity monomer and application of sesquiterpene isomer derivative and high-purity monomer in antitumor drugs
A technology of sesquiterpenes and isomers, which is applied in the field of compound preparation and monomer compound separation, can solve the problems such as the separation of three configurations of celeryne, and achieve high purity and good separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In a typical embodiment of the present invention, a method for preparing sesquiterpene isomer derivatives with efficient conversion and high-purity monomers is provided, and the preparation method at least includes:
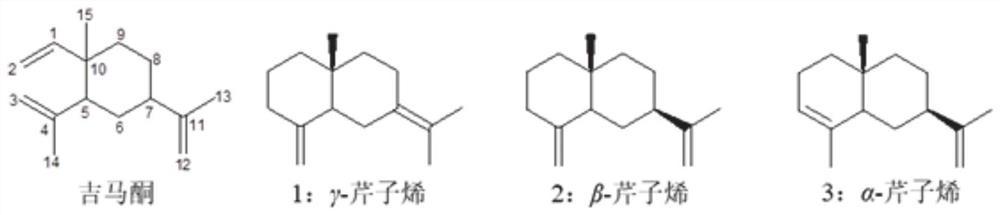
[0029] Gemacerone is converted into celeryne, and separated and extracted using high-speed countercurrent chromatography and preparative HPLC to obtain three configuration monomers of celeryne; in the high-speed countercurrent chromatography, the solvent system is Ag-containing + of n-hexane / methanol / water.
[0030] Described gemaconone is converted into the concrete method of apigenene as:
[0031] Add silica gel and phosphoric acid aqueous solution to gemaconone solution, and react at high temperature.
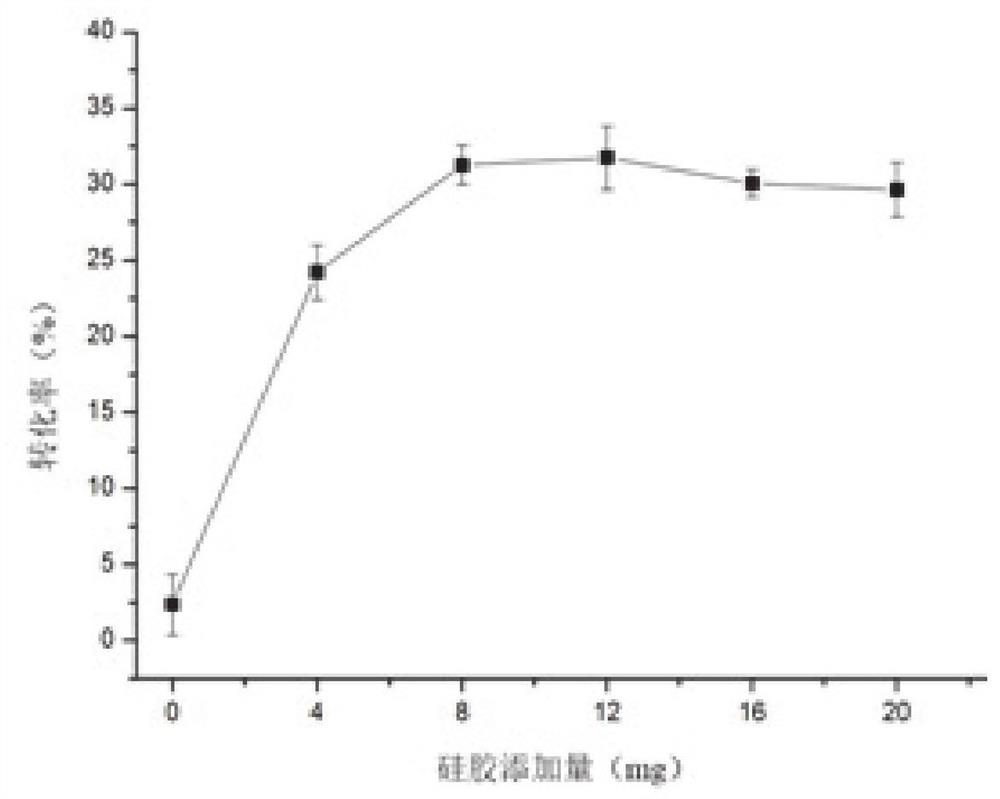
[0032] Wherein, the volume mass of the gemacerone and silica gel is 100-500 μL: 1-20 mg, such as 200 μL: 4 mg, 200 μL: 8 mg, 200 μL: 12 mg, 200 μL: 16 mg and 200 μL: 20 mg;
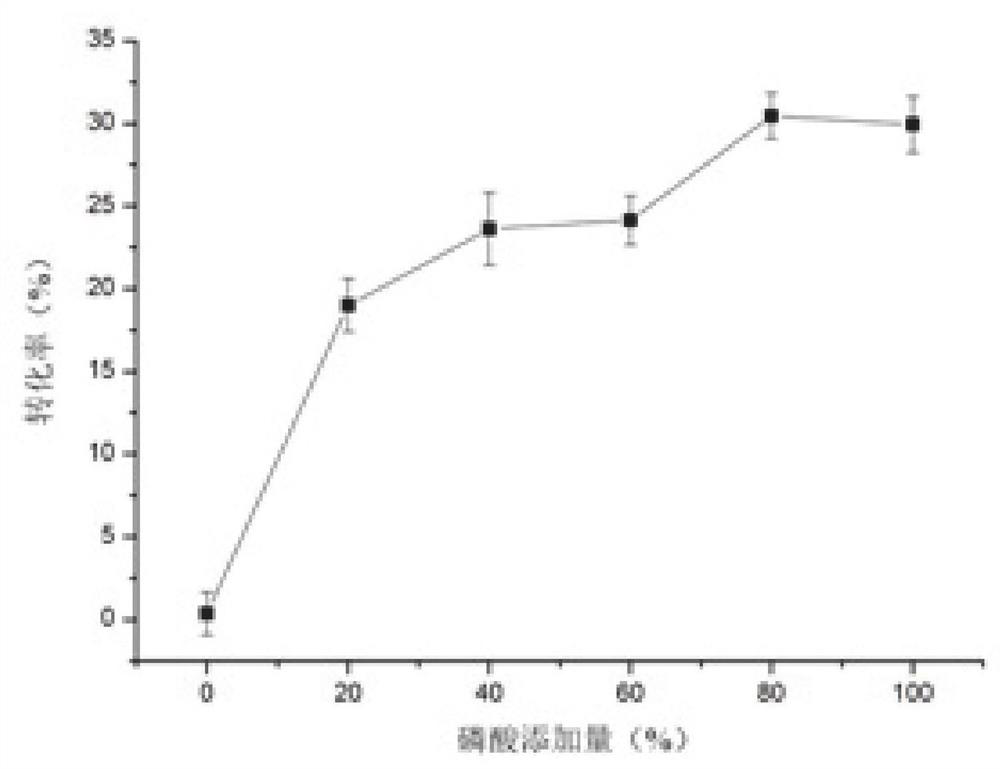
[0033] The concentration of the phosphoric acid aqueous solution is controlle...
Embodiment
[0054] 1. Single factor experiment on the conversion of gemmazone to celrenene isomers
[0055] 1. Process flow:
[0056] Use a pipette gun to accurately draw 200 μL of a crude sample of gemmaquinone into a stoppered test tube, add different amounts of silica gel (200-300 mesh) as a catalyst for the reaction, and react at a certain temperature for a certain period of time in different pH environments. After the reaction was completed, the reaction solution was transferred to a centrifuge tube, centrifuged at 8000r / min for 10min, and 10μL of the supernatant was accurately measured and diluted with 1mL of methanol, and then detected by HPLC.
[0057] 2. Process optimization:
[0058] Select different silica gel addition amounts (0, 4, 8, 12, 16 and 20mg), add 5 μ L of phosphoric acid aqueous solution of different concentrations (0%, 20%, 40%, 60%, 80% and 100%), different Reaction time (10, 20, 30, 40, 50, and 60min), different reaction temperatures (30, 50, 70, 90, 110, and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com