Oxime ether (ester) compound as well as preparation method and application thereof
A technology of compound and oxime ether, applied in the field of pathogenic bacteria control, to achieve the effect of novel structure, broad-spectrum bactericidal activity and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of compound 2-fluorobenzaldehyde O-(1,2,3,4-tetrahydroquinoline-1-carbonyl)oxime (I-01)
[0053]
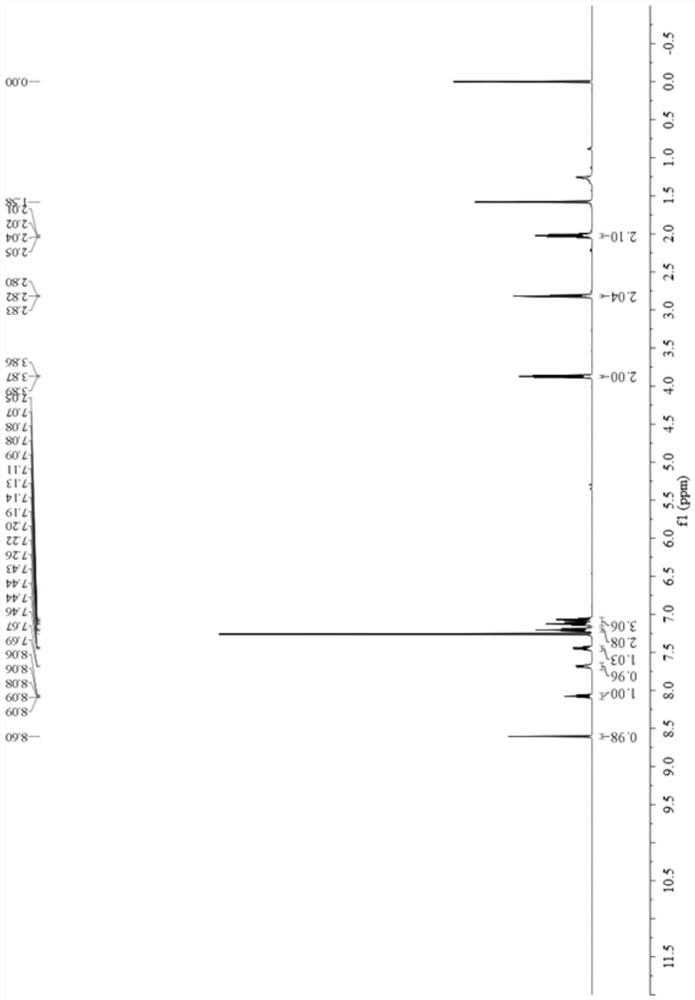
[0054] Add 0.6957 g (5 mmol) of 2-fluorobenzaldehyde oxime and 10 mL of dichloromethane into a 25 mL single-necked bottle, and stir at room temperature. Then, a solution of 0.9783 g (5 mmol) of 1,2,3,4-tetrahydroquinoline-1-formyl chloride in 5 mL of dichloromethane was added dropwise thereto, and the reaction was continued for 300 min after the dropwise addition was completed. Purified by column chromatography (petroleum ether: ethyl acetate = 15:1), after drying, 0.9695 g of light yellow solid was obtained, yield 65%, melting point: 98-100°C, 1 H NMR (500MHz, CDCl 3 )δ8.60(s,1H),8.09–8.06(m,1H),7.68(d,J=8.1Hz,1H),7.45(m,1H),7.20(t,J=7.6Hz,2H), 7.09 (m, 3H), 3.88–3.86 (m, 2H), 2.82 (t, J=6.6Hz, 2H), 2.04–1.99 (m, 2H).
Embodiment 2
[0055] Example 2: Preparation of compound 3-methylbenzaldehyde O-(1,2,3,4-tetrahydroquinoline-1-carbonyl)oxime (I-10)
[0056]
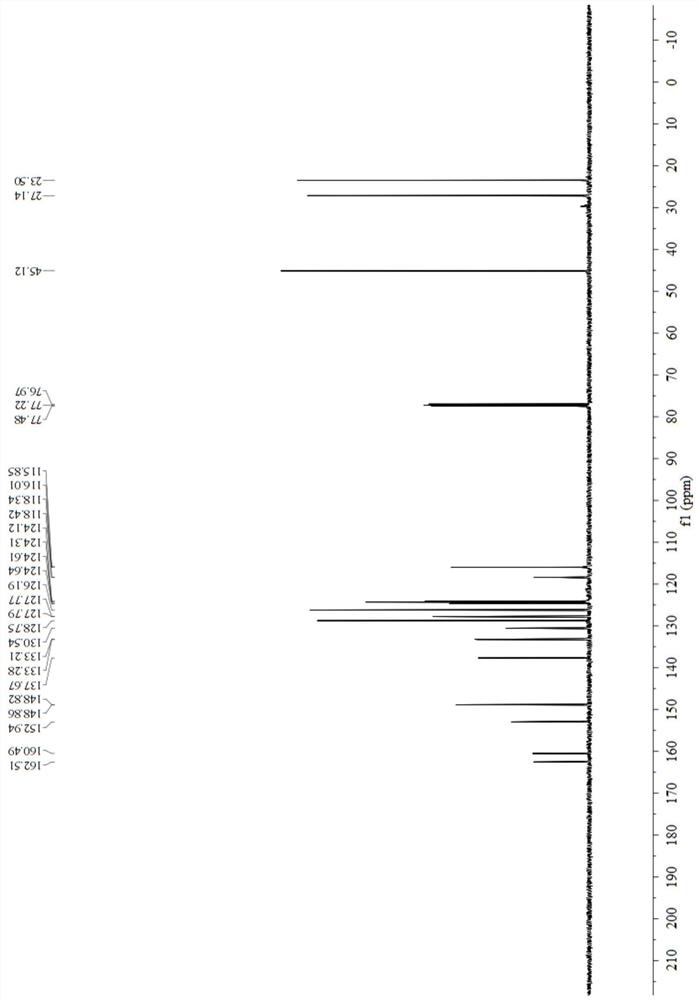
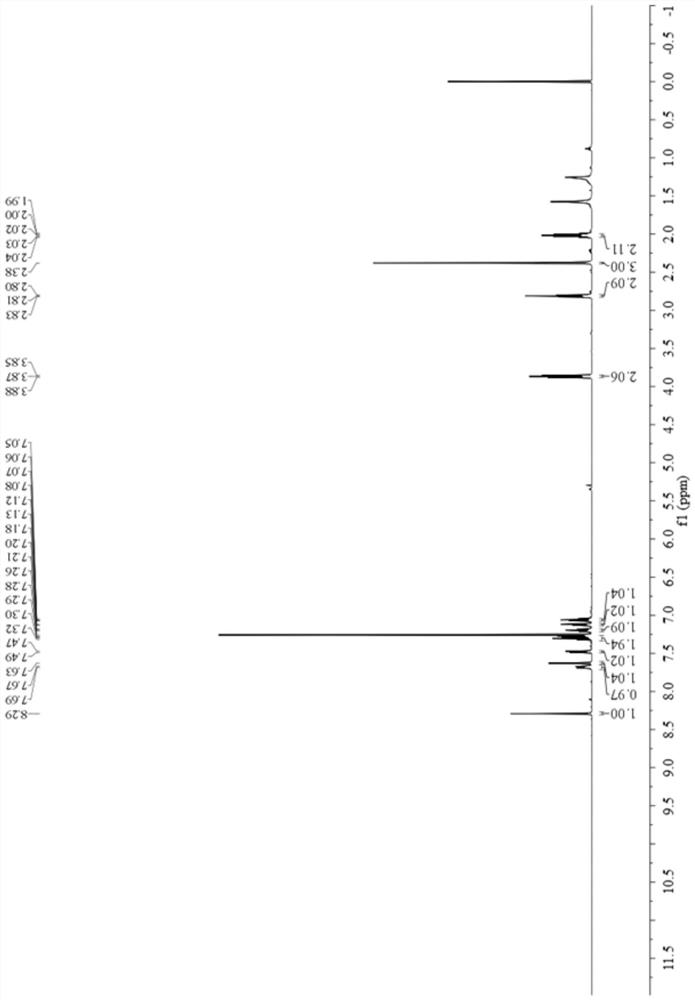
[0057] Add 0.6759 g (5 mmol) of 3-methylbenzaldehyde oxime, 0.5060 g (5 mmol) of triethylamine, and 10 mL of toluene into a 25 mL single-necked bottle at room temperature. Then, a 5 mL toluene solution of 0.9783 g (5 mmol) of 1,2,3,4-tetrahydroquinoline-1-carbonyl chloride was added dropwise thereto, and the reaction was continued for 5 minutes after the dropwise addition was completed. Purified by column chromatography (petroleum ether: ethyl acetate = 10:1), and dried to obtain 0.8831 g of a yellow solid, yield 60%, melting point: 88-90°C, 1 H NMR (500MHz, CDCl 3 )δ8.29(s,1H),7.68(d,J=8.2Hz,1H),7.63(s,1H),7.48(d,J=7.4Hz,1H),7.30(dd,J=14.6,7.2 Hz, 2H), 7.20(t, J=7.7Hz, 1H), 7.13(d, J=7.0Hz, 1H), 7.06(dd, J=11.0, 3.7Hz, 1H), 3.88–3.85(m, 2H ), 2.81(t, J=6.6Hz, 2H), 2.38(s, 3H), 2.03–2.00(m, 2H), 13 C NMR (125MHz, CDCl 3 )δ 155.38, 153.19, 138...
Embodiment 3
[0058] Example 3: Preparation of compound 3-phenoxybenzaldehyde O-(1,2,3,4-tetrahydroquinoline-1-carbonyl)oxime (I-16)
[0059]
[0060] Add 1.0662 g (5 mmol) of 3-phenoxybenzaldoxime, 0.3955 g (5 mmol) of pyridine, and 10 mL of ethyl acetate into a 25 mL single-necked bottle, and stir at -20°C. Then 1,2,3,4-tetrahydroquinoline-1-formyl chloride 1.1739g (6mmol) in 5mL ethyl acetate solution was added dropwise thereto, and the reaction was carried out for 120min after the dropwise addition was completed. Purified by column chromatography (petroleum ether: ethyl acetate = 10:1), and dried to obtain 1.2290 g of a yellow solid, yield 66%, melting point: 76-78 °C, 1 H NMR (500MHz, CDCl 3 )δ8.27(s,1H),7.65(d,J=8.0Hz,1H),7.48(d,J=7.7Hz,1H),7.36(dt,J=15.3,8.0Hz,4H),7.18( t,J=7.9Hz,1H),7.11(q,J=7.1Hz,3H),7.03(dd,J=23.9,7.6Hz,3H),3.85–3.83(m,2H),2.80(t,J =6.6Hz, 2H), 2.01(q, J=6.4Hz, 2H), 13 C NMR (125MHz, CDCl 3 )δ157.87, 156.70, 154.80, 153.03, 137.71, 132.10, 130.58, 130.25, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com