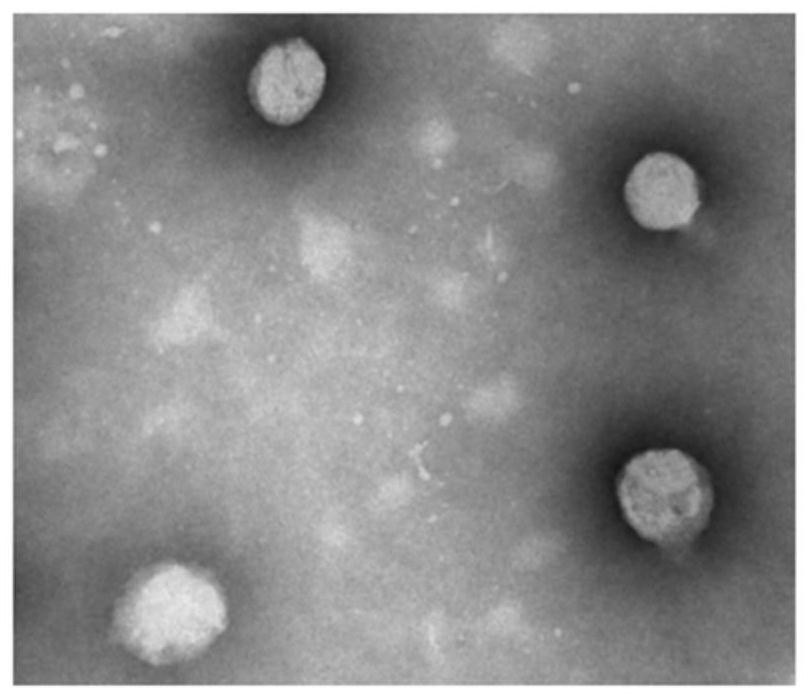
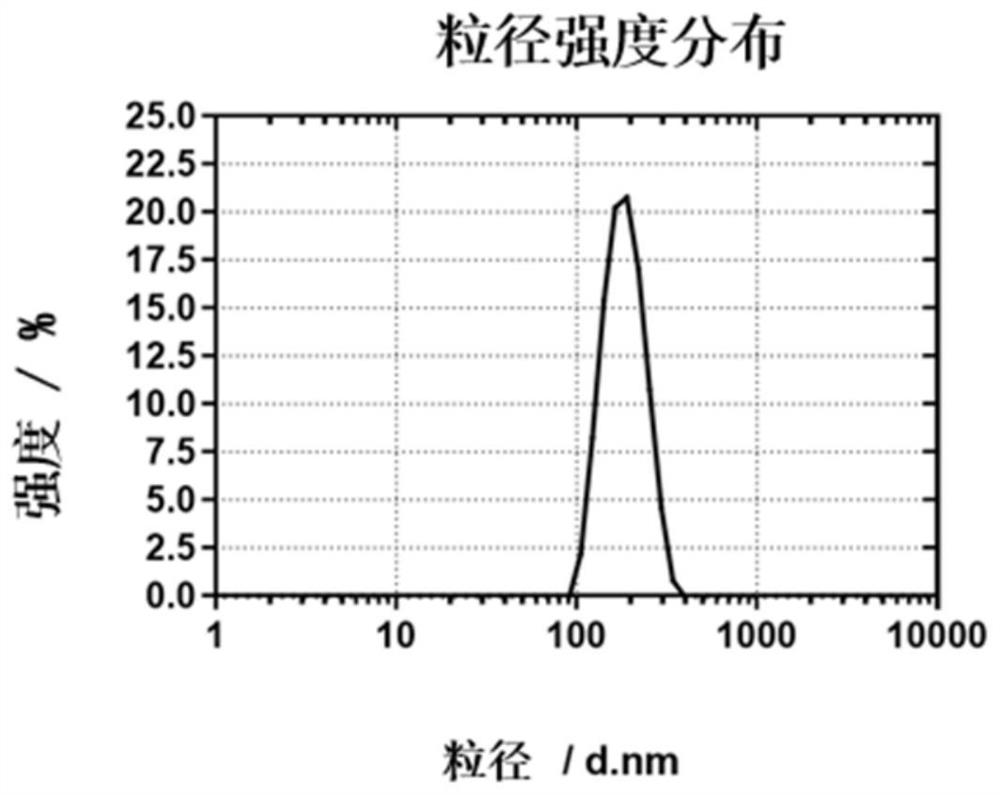
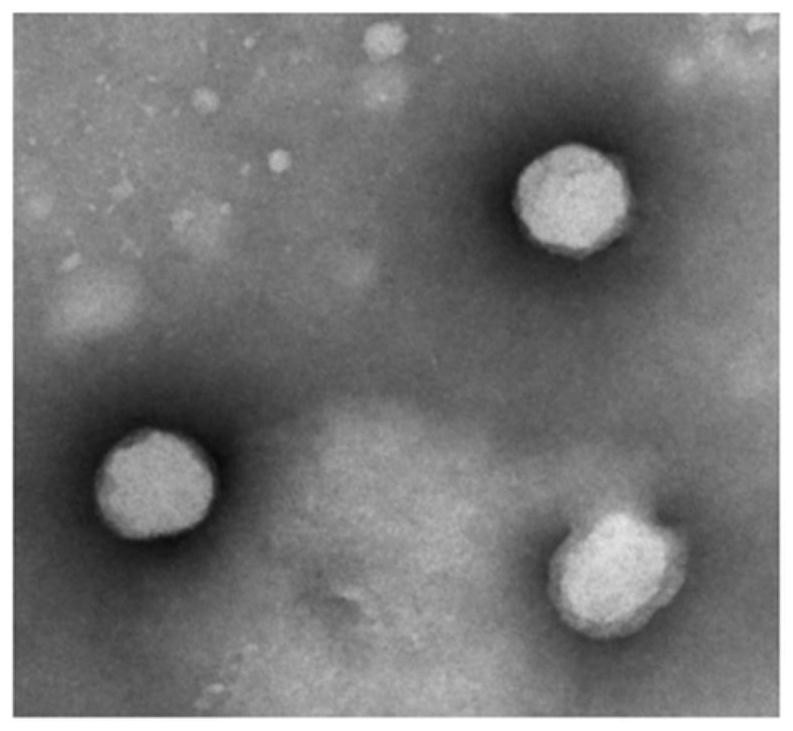
Squalene chidamide prodrug self-assembled nanoparticles, and preparation method and application thereof
A technology of self-assembled nanoparticles and chidamide, applied in the field of medicine, can solve the problems of low tumor microenvironment permeability, poor targeting, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation process of squalene-based chidamide prodrug molecule (SQ-CHI) comprises the following steps:
[0058] Weigh 1,1',2-Tris-norsqualenoyl acid (1,1',2-Tris-norsqualenoyl acid, SQ-COOH) 100mg and add 1mL DMF to dissolve evenly, and gradually add N-hydroxysuccinimide ( NHS) 30 mg and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) 100 mg were reacted at room temperature for 30 min under nitrogen protection. Accurately weigh 100 mg of Chidaniline and dissolve it in 1 mL of DMF, add it dropwise to the above reaction system, continue to stir and react at room temperature under nitrogen protection for 48 h, and adopt flash column chromatography (eluent is dichloromethane:methanol = 99:1; 95:5) for purification. Results The SQ-CHI prodrug molecule was successfully prepared.
Embodiment 2
[0060] The preparation process of squalene-based chidamide prodrug molecule (SQ-CHI) comprises the following steps:
[0061] Weigh 1,1',2-Tris-norsqualenoyl acid (1,1',2-Tris-norsqualenoyl acid, SQ-COOH) 200mg and add 1mL DMF to dissolve evenly, and gradually add N-hydroxysuccinimide ( NHS) 60 mg and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) 50 mg, reacted for 120 min at room temperature under nitrogen protection. Accurately weigh 100 mg of Chidaniline and dissolve it in 1 mL of DMF, add it dropwise to the above reaction system, continue to stir and react at room temperature under nitrogen protection for 96 h, and adopt flash column chromatography (eluent is dichloromethane:methanol = 95:5; 85:5) for purification. Results The SQ-CHI prodrug molecule was successfully prepared.
[0062] Both Example 1 and Example 2 successfully prepared SQ-CHI prodrug molecules, and the prodrug molecules prepared in Example 1 were preferred for subsequent research.
Embodiment 3
[0064] The preparation process of folic acid-polyethylene glycol-squalene (FA-PEG-SQ) small molecule ligand comprises the following steps:
[0065] Accurately weigh 100mg of SQ-COOH and add 100μLDMF to dissolve evenly, gradually add 30mg of NHS and 100mg of EDCI to the reaction system, and react for 30min at room temperature under nitrogen protection. Accurately weigh FA-PEG-NH 2 100 mg of the powder was dissolved in 100 μL of DMF, and added dropwise to the above reaction system, and the reaction was continued with stirring at room temperature for 48 h under nitrogen. At the end of the reaction, use an ultrafiltration centrifuge tube (molecular cut-off 3KDa), and centrifuge at a high speed of 10000rpm for 30min. Results The SQ-PEG-FA complex was successfully prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com