Fluorescent probe for detecting pyroglutamamide aminopeptidase I as well as preparation method and application of fluorescent probe
A technology of pyroglutamylamino and fluorescent probes, applied in the field of fluorescent probes for detecting pyroglutamyl aminopeptidase I and its preparation, achieving the effects of excellent photophysical properties, low cost, and strong penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
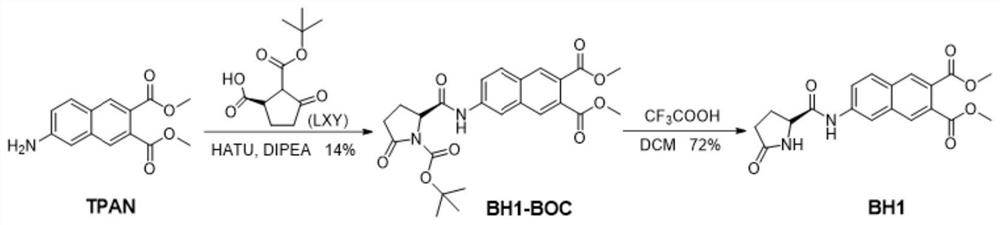
[0036] A kind of synthetic method for detecting the fluorescent probe of pyroglutamyl aminopeptidase I, comprises the following steps:
[0037] Mix LXY, HATU and DIPEA, completely dissolve in dry dichloromethane solution, stir and react at 0°C; then add TPAN solution, further stir and react at room temperature, and then purify to obtain a deep red solid BH1-BOC;
[0038] Dissolve BH1-BOC in anhydrous dichloromethane, add the prepared trifluoroacetic acid solution under the condition of ice-salt bath, and carry out stirring reaction at room temperature after the dropwise addition; spin to dry the solvent, then add dichloromethane to it, repeat Several times, until all the trifluoroacetic acid was spin-dried; the crude product was purified by TM to obtain a red solid BH1.
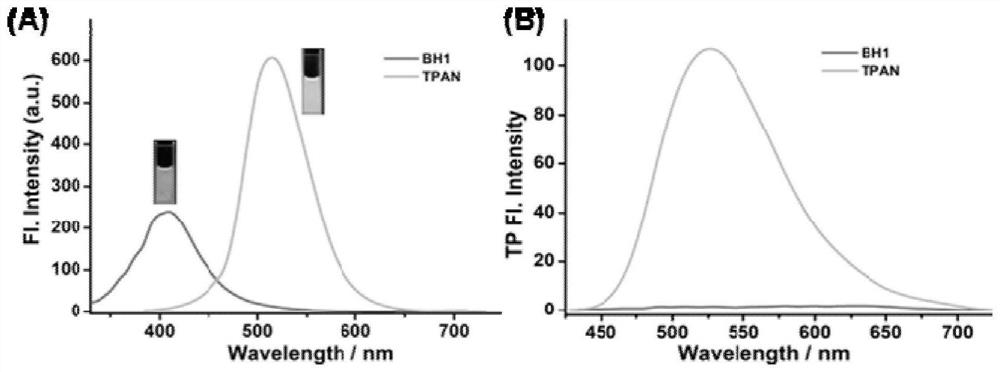
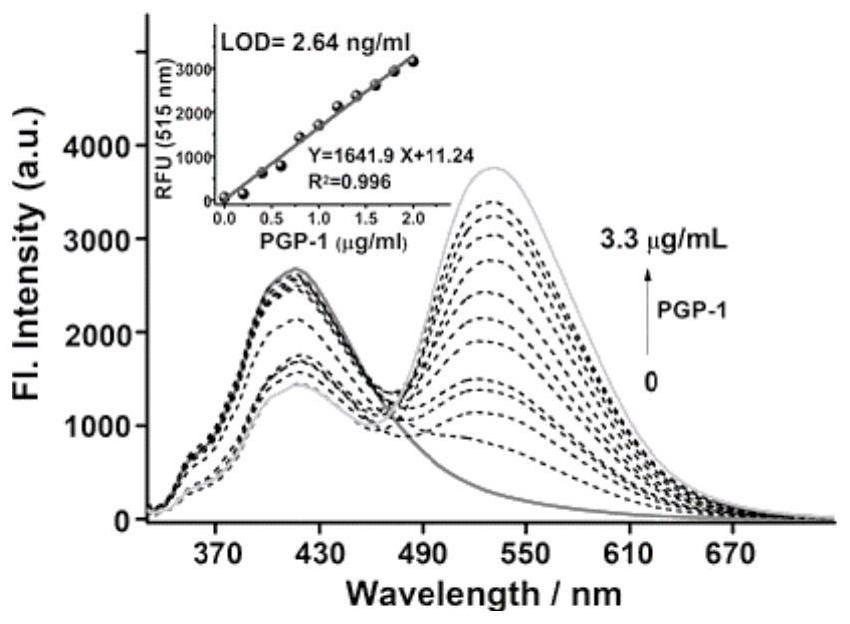
[0039] The ability of the probe to analyze and detect PGP-1 was evaluated in vitro; with 350nm as excitation, after adding PGP-1, the fluorescence spectrum of the probe will change obviously. Probe BH1 exhib...
Embodiment 1
[0054] 1. Synthesis of Dimethyl(R)-6-(1-(tert-butoxycarbonyl)-5-oxopyrrolidine-2-carboxamido)naphthalene-2,3-dicarboxylate(BH1-BOC)
[0055] Add dimethyl 6-aminonaphthalene-2,3-dicarboxylate (LXY; 115mg, 0.5mmol), 2-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluroniumhexafluorophosphate (HATU; 190mg, 0.5mmol) and N,N-Diisopropylethylamine (DIPEA; 200μL, 1.25mmol), which were completely dissolved in dry dichloromethane solution (25mL), and the above reactants were stirred at 0°C for 30 minutes. Then, post-TPAN (130 mg, 0.5 mmol) dissolved in dichloromethane (5 mL) was further added, followed by further stirring at room temperature for 4 hours. After the reaction was over, distilled under reduced pressure and spin-dried the solvent, the crude product was purified by silica gel chromatography, using petroleum ether / ethyl acetate (15 / 1, v / v) as eluent to obtain a dark red solid BH1-BOC ( 70 mg), yield: 14%. BH1-BOC: 1 H NMR (500MHz, DMSO-d 6 )δppm: 10.80(s, 1H), 8.44(s, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com