Universal human-derived stem cell suitable for allograft and construction method thereof
A stem cell and cell technology, applied in the medical field, can solve problems such as immune rejection, achieve low immunogenicity, and avoid the effect of allogeneic immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
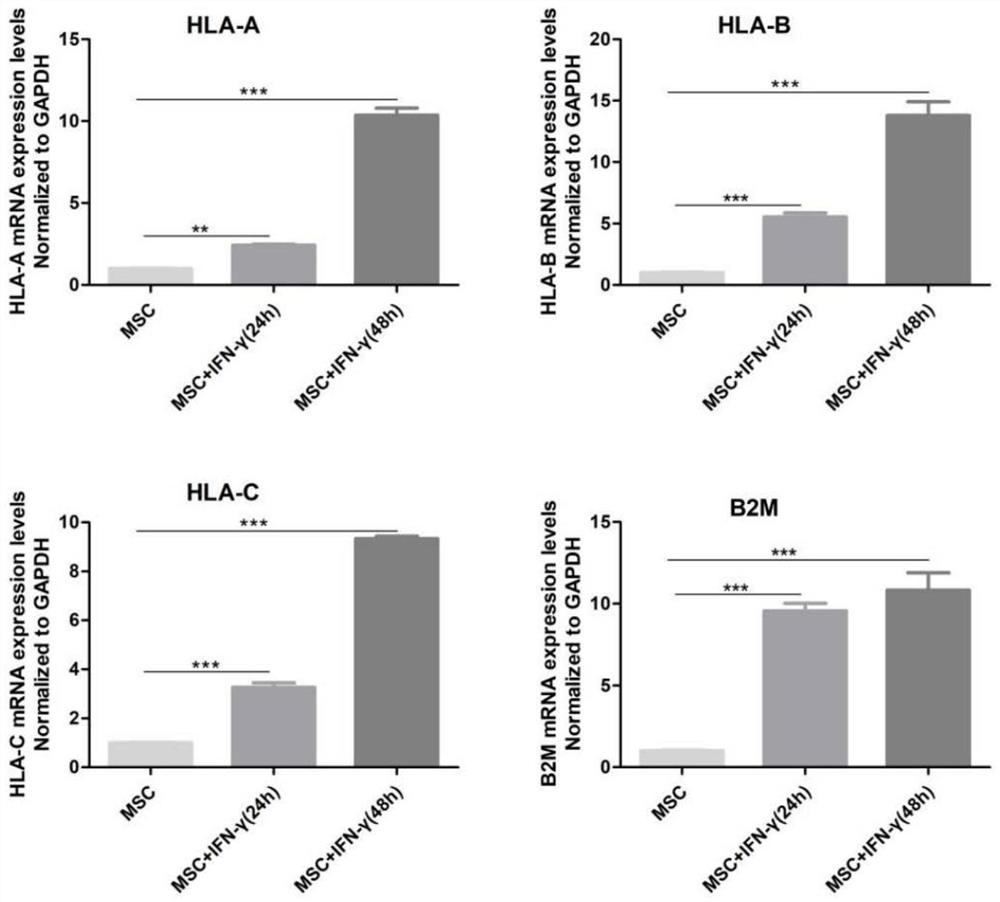
[0093] Example 1: IFN-γ Up-regulates HLA-I Gene Expression of MSCs
[0094] This example proves through specific experiments that IFN-γ can up-regulate the expression of the HLA-I gene of MSCs.
[0095] 1.1 Cell culture and treatment
[0096] MSC culture conditions: L-DMEM medium (containing 10% fetal bovine serum), 5% CO2, 37°C constant temperature culture. 24 hours before treatment, MSCs were treated with 5 × 10 5 Each well was seeded into a 6-well plate for culture, and the cell confluency reached 60%-70% the next day.
[0097] The cultured MSCs were divided into three groups, the first group was blank control, the second group added IFN-γ to the medium at a concentration of 100ng / ml, and continued to culture for 24 hours; the third group, IFN-γ was added at a concentration of 100ng / ml -γ was added to the culture medium and continued to culture for 48 hours; the cells were collected for subsequent processing such as RNA extraction.
[0098] 1.2 MSC RNA extraction and fl...
Embodiment 2
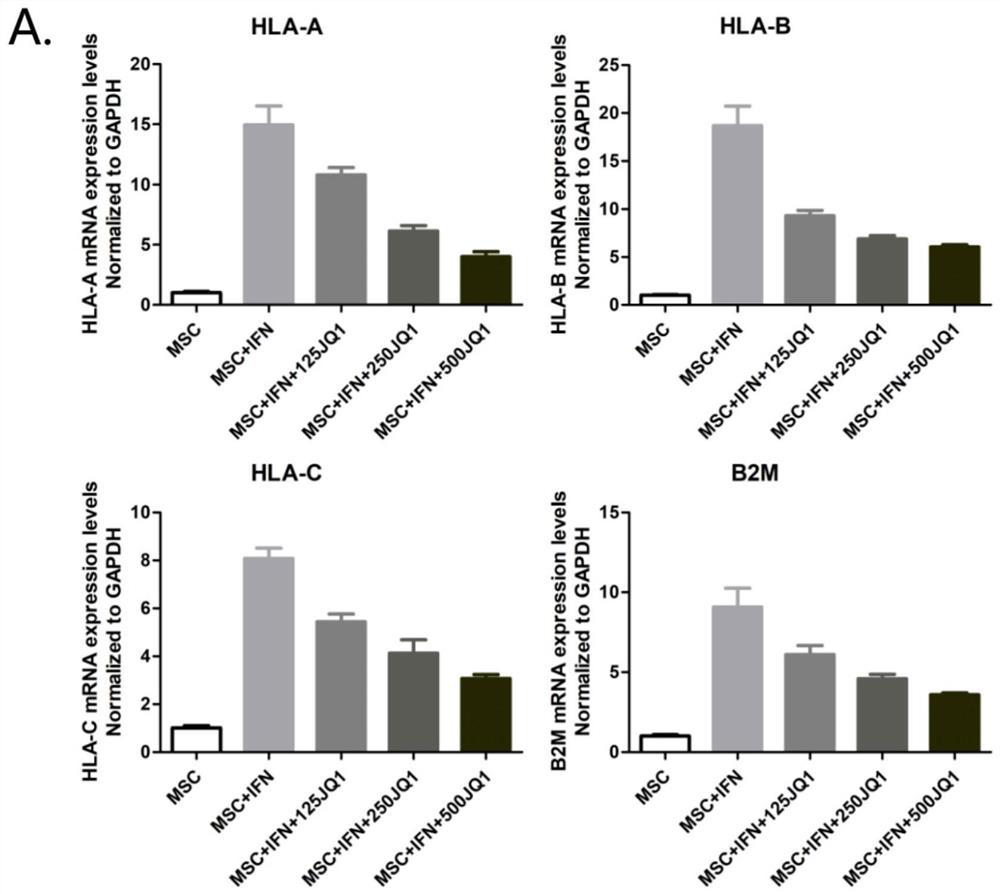
[0122] Example 2: Experiments involving the participation of enhancers in the process of IFN-γ stimulating HLA-I expression
[0123] In this example, JQ1 (purchased from: abcam; product number: ab146612; batch number: APN15092-1-1; an enhancer inhibitor) with three concentrations of 125, 250 and 500nmol / ml were used to pretreat MSCs for 2 hours, and then Add 100ng / ml IFN-γ to treat MSC, continue to culture for 48 hours, collect cells and extract RNA for qPCR to detect the expression of HLA-I related genes, and set the corresponding MSC blank control, and MSC blank control plus IFN-γ treatment, A total of five groups of MSCs were compared and detected by q-PCR and flow cytometry respectively. The results are shown in Figure 3. For the specific process and sequence of q-PCR, refer to the process and conditions of Example 1. For the q-PCR results, see Figure 3A , is the expression level of HLA-A, HLA-B, HLA-C and B2M of MSC in the five groups; the results of flow cytometry are s...
Embodiment 3
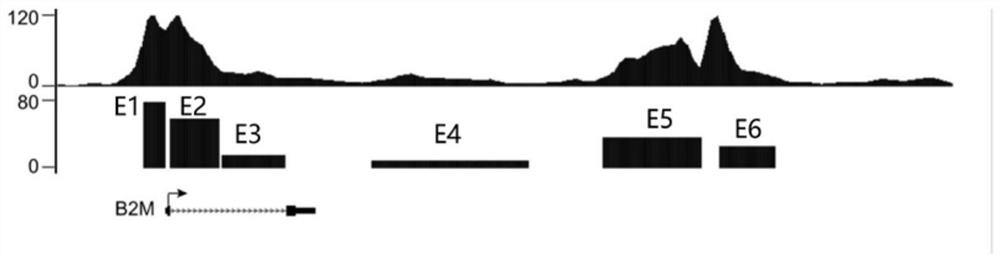
[0136] Example 3, IFN-γ has a promoting effect on the opening and activation of the enhancer near the B2M gene
[0137] In order to further understand whether there are enhancers involved in the vicinity of HLA-I related genes after IFN-γ stimulation, and whether it is directly related to the expression of that gene, we performed H3K27ac ChIP-Seq analysis and bioinformatics analysis.
[0138] 1. The process of H3K27ac ChIP-Seq analysis is as follows:
[0139] 1 x 10 at room temperature 7 The non-IFN-γ-stimulated MSCs and IFN-γ-stimulated MSCs were respectively fixed with 1% formaldehyde for 10 minutes to obtain DNA-protein crosslinks. After sonicating for 1.5 hours to obtain 200-300bp chromatin fragments, mix with H3K27ac antibody (H3K27ac is a recognized active enhancer marker protein, and use H3K27ac antibody to capture all enhancer DNA bound to H3K27ac protein) at 4°C After overnight incubation, antibody-chromatin complexes were obtained by incubating with Dynabeads Prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com