Helicobacter pylori compound antigen, antibody, preparation method and application
A technology of Helicobacter pylori and Helicobacter pylori, which is applied in the field of biomedicine, can solve the problems of poor colonization inhibition effect and the effect needs to be improved, and achieve the effects of optimizing amino acid sequence, increasing potency, increasing concentration and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
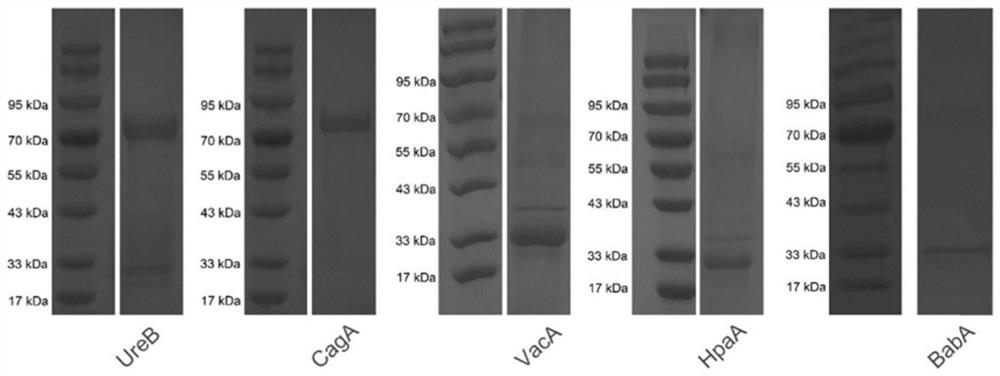
[0048] This embodiment provides an Hp pentavalent composite antigen, which is composed of five antigen proteins, urease (UreB), vacuolar cytotoxin (VacA), cytotoxic gene A (CagA), adhesin HpaA and adhesin BabA; The amino acid sequences of the five antigenic proteins are respectively shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3, SEQ ID No.4 and SEQ ID No.5 after optimization; wherein, the sequence optimization includes The code preference of Escherichia coli and the sequence conservation of Helicobacter pylori are optimized, and the polypeptide sequence CTB is added to the N-terminus or C-terminus of the HpaA antigen protein. The CTB sequence is shown in SEQ ID No. 11; Add a protein expression tag to the C-terminus.
[0049] The specific preparation method is as follows:
[0050] Expression of S1 and Hp complex antigens
[0051] The nucleotide sequences corresponding to the five antigen proteins of urease (UreB), vacuolar cytotoxin (VacA), cytotoxin gene A (CagA), adhesin H...
Embodiment 2
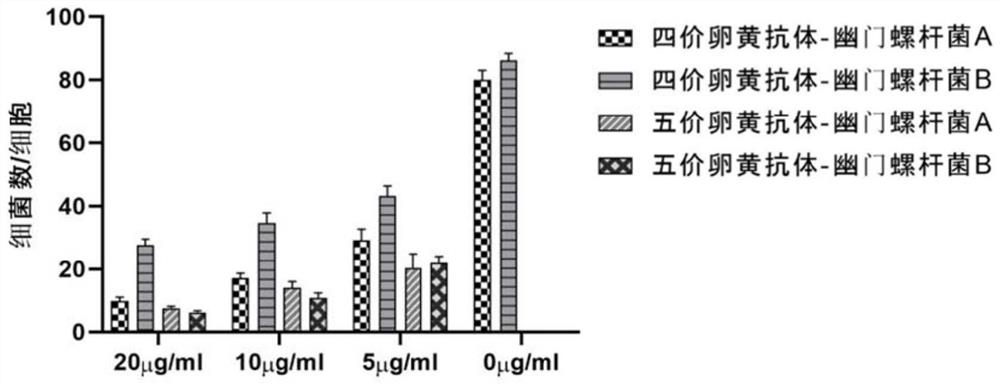
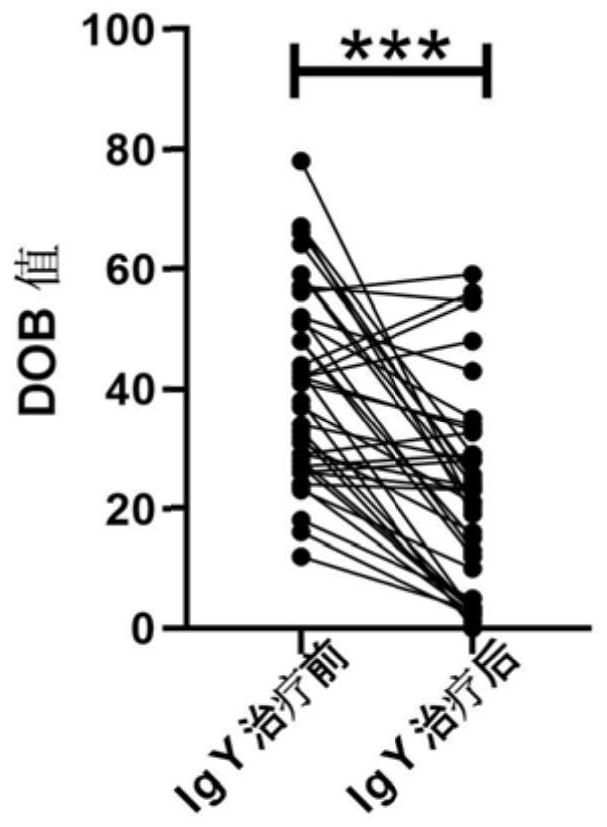
[0069] This example provides an anti-Helicobacter pylori yolk antibody. The pentavalent composite antigen prepared in Example 1 is used to induce immunization of hens, and then a yolk antibody containing anti-five Hp antigens is obtained. The specific preparation steps are as follows:
[0070] (1) five kinds of Hp combined antigens are emulsified with adjuvant; the adjuvant is Freund's complete adjuvant;
[0071] (2) Immunization of laying hens: 10-40-week-old healthy laying hens are selected for isolation and rearing, and subcutaneous injection is carried out. Each hen is injected with 2-6 injection points. 80μg~200μg / chicken is preferred, 100μg / chicken is used in this example, after the first immunization, booster immunization is performed once every two weeks;
[0072] (3) collecting eggs produced by the last immunization, namely obtaining eggs containing yolk antibodies against five Hp antigens;
[0073] (4) separating and purifying the egg containing the egg yolk antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com