New application of Apiosylskimmin in preparation of medicine for treating cholestasis
A technology for cholestasis and drugs, applied in the field of preparation of drugs for treating cholestasis, can solve problems such as lack of expression of CYP2B, increase of serum bilirubin level, etc., and achieve broad application prospects and good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
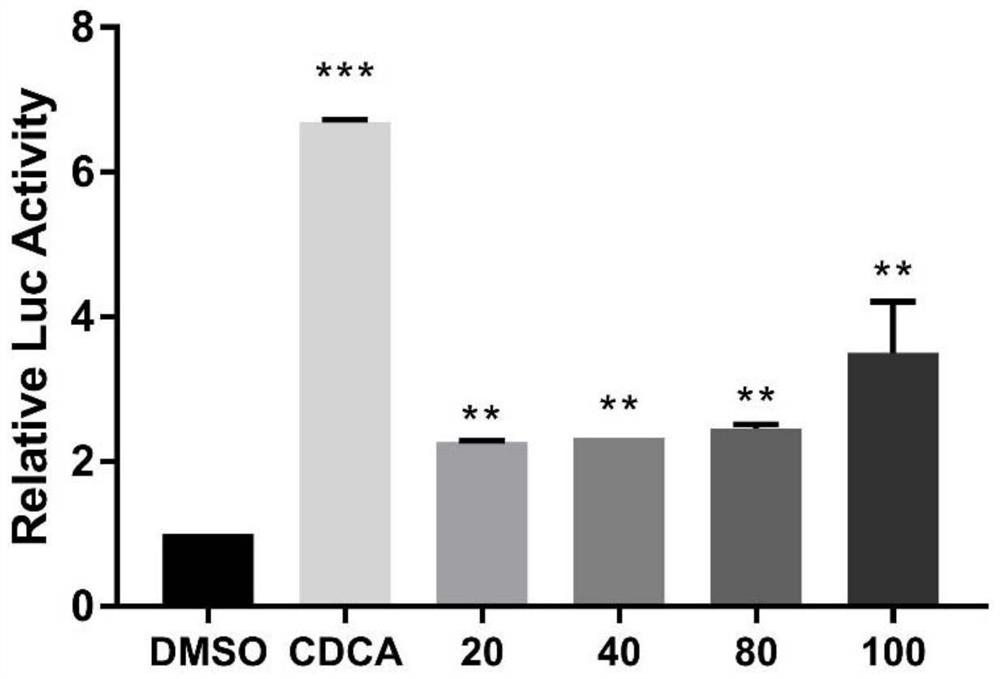
[0047]Example 1 Determination of FXR agonistic activity
[0048] Establish a dual-luciferase reporter gene assay for FXR agonist screening cell verification method, the specific experimental steps are as follows:
[0049] 1. HepG2 cells were inoculated in a 96-well plate at a concentration of 15,000 cells / well, cultured in complete DMEM medium for 24 hours, and then transfected when the cells grew to about 70%; the transfection reagent X-tremegene (Roche), overexpressed in advance Plasmid FXR, reporter gene plasmid BSEP, renilla luciferase plasmid, and Opti-MEM were cultured based on a room temperature of 15-25 ° C for 20 minutes; the transfection mixture was prepared in proportion, each 100 μL Opti-MEM medium contained 1 μg of each plasmid, 4 μL of transfection reagent, the ratio of plasmid and transfection reagent is 1:2, mix gently; take out the 96-well plate, add 10 μL of transfection mixed reagent to each well, and continue to incubate for 24 hours; after 24 hours, add 20...
Embodiment 2
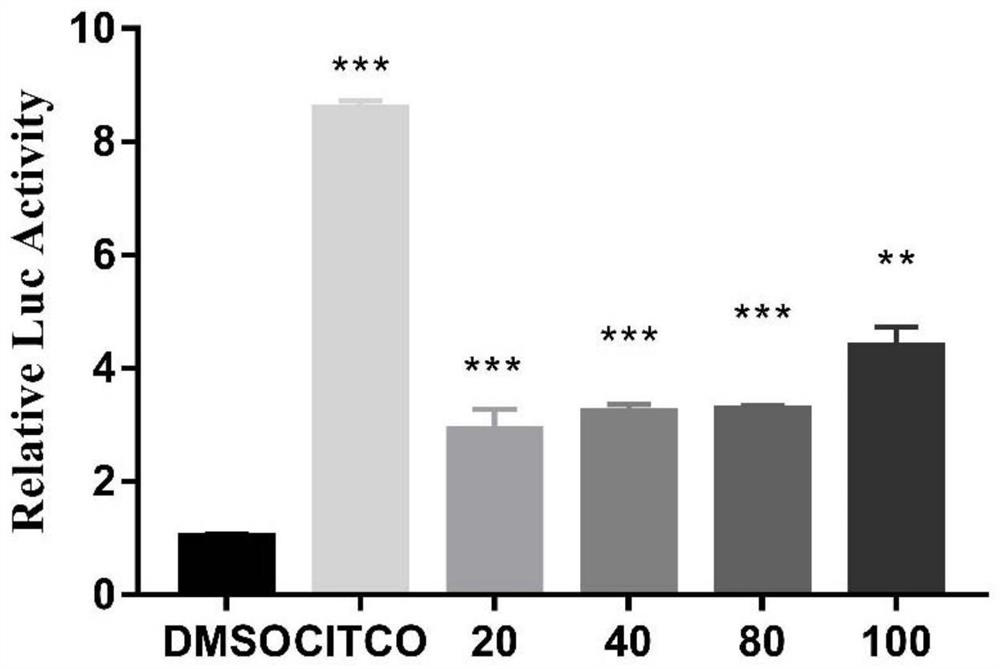
[0052] Example 2 Determination of CAR agonistic activity
[0053] Establish a dual-luciferase reporter gene assay for CAR agonist screening cell verification method, the specific experimental steps are as follows:
[0054] 1. HepG2 cells were inoculated in a 96-well plate at a concentration of 15,000 cells / well, cultured in complete DMEM medium for 24 hours, and then transfected when the cells grew to about 70%; the transfection reagent X-tremegene (Roche), overexpressed in advance Plasmid CAR, reporter gene plasmid CYP2B6 and Renilla luciferase plasmid, and Opti-MEM culture were based on a room temperature of 15-25°C and equilibrated for 20 minutes; the transfection mixture was prepared in proportion, each 100 μL Opti-MEM medium contained 1 μg of each plasmid, Transfection Reagent 4 μL of plasmid and transfection reagent at a ratio of 1:2, mix gently; take out the 96-well plate, add 10 μL of transfection mixed reagent to each well, and continue to incubate for 24 hours; add 2...
Embodiment 3
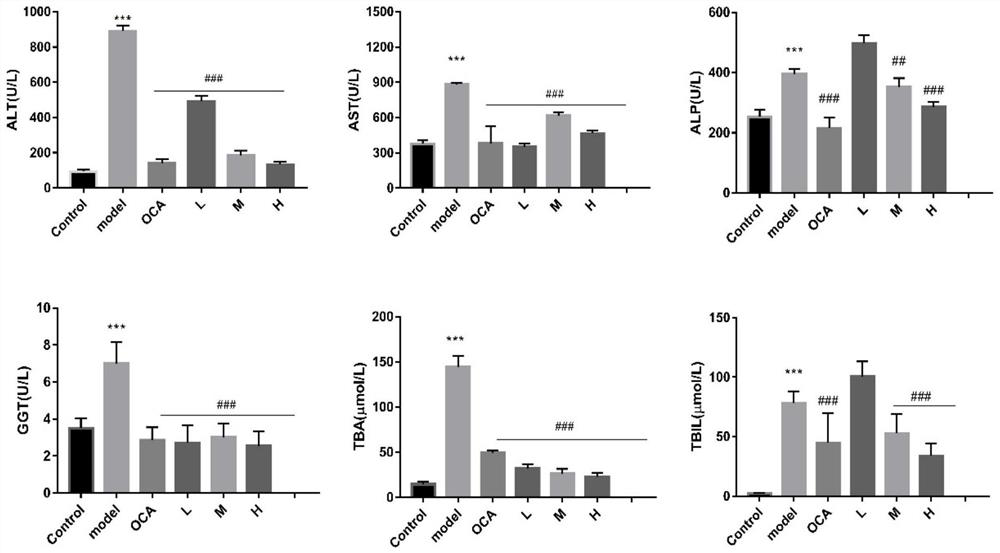
[0057] Example 3 Apiosylskimmin's therapeutic effect on cholestasis mice
[0058] 1. Experimental materials
[0059] Male C57BL / 6 mice, weighing 18±2g, were provided by the Gansu Provincial Institute for Drug Control; they were housed in separate cages in plastic cages, free to eat and drink for 7 days, and allowed them to adapt to the environment and quarantine.
[0060] 2. Experimental method
[0061] In this experiment, 1-naphthyl isothiocyanate (ANIT) was used to establish a mouse model of acute cholestasis, and the therapeutic effect of Apiosylskimmin on this model mouse was studied.
[0062] 2.1 Experimental grouping
[0063] The mice were randomly divided into normal control group, model control group, low-dose Apiosylskimmin group, middle-dose Apiosylskimmin group, high-dose Apiosylskimmin group, and positive drug obeticholic acid (OCA) group, with 8 mice in each group.
[0064] 2.2 Administration method
[0065] Apiosylskimmin low-dose group, Apiosylskimmin middle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com