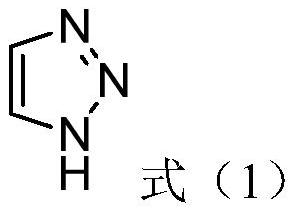
Synthesis method for preparing 1H-1, 2, 3-triazole by continuous flow
A synthesis method and 1H-1 technology, applied in organic chemistry and other directions, can solve the problems of high safety risk and low production efficiency, and achieve the effect of reducing safety risk, good product quality and improving catalyst utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
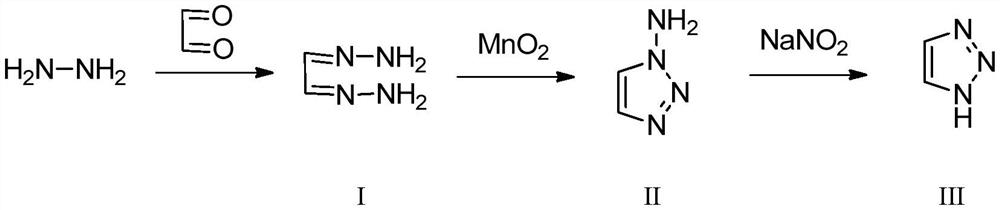
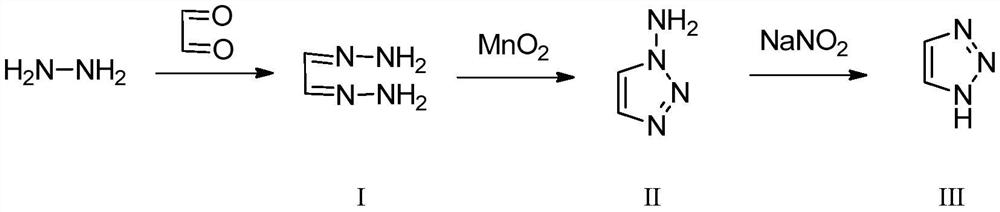
[0042] This embodiment provides a preparation method of 1H-1,2,3-triazole, which specifically includes the following steps:
[0043] 1) Take 91.2g of 80% hydrazine hydrate solution and pump it into the preheating equipment at a flow rate of 0.5mL / min for preheating. The preheating temperature is 35°C. 100g of aqueous solution is pumped into the reactor at a flow rate of 0.5mL / min, the temperature of the reactor is set at 30°C, and then the two react, and the intermediate I is collected through the condenser at room temperature;
[0044] 2) Add 1000mL ethanol to intermediate I and mix it evenly, pump it into the reaction column tube containing manganese dioxide (1.0 equivalent) at a flow rate of 0.5mL / min, and inject oxygen into it at a speed of 10mL / min Carry out the oxidation reaction in the above-mentioned continuous reaction column, and the reaction temperature is set at 25° C. to obtain intermediate II;
[0045] 3) Put the obtained intermediate II and 30% hydrochloric aci...
Embodiment 2
[0048] This embodiment provides a preparation method of 1H-1,2,3-triazole, which specifically includes the following steps:
[0049] 1) Take 91.2g of 80% hydrazine hydrate solution and pump it into the preheating equipment at a flow rate of 1mL / min for preheating. The preheating temperature is 40°C. 100g was pumped into the reactor at a flow rate of 0.5mL / min, and the temperature of the reactor was set at 40°C, and then the two reacted, and the intermediate I was collected by cooling down to room temperature through the condenser;
[0050] 2) Add 1000mL of ethanol to Intermediate I and mix it evenly, pump it into the reaction column tube containing manganese dioxide (1.0 equivalent) at a flow rate of 1mL / min, inject oxygen into the reaction column at a rate of 20mL / min Oxidation reaction is carried out in the above-mentioned continuous reaction column, and the reaction temperature is set at 25° C. to obtain intermediate II;
[0051] 3) Put intermediate II and 30% hydrochloric...
Embodiment 3
[0054] This embodiment provides a preparation method of 1H-1,2,3-triazole, which specifically includes the following steps:
[0055] 1) Take 91.2g of 80% hydrazine hydrate solution and pump it into the preheating equipment at a flow rate of 1mL / min for preheating. The preheating temperature is 60°C. 100g was pumped into the reactor at a flow rate of 1mL / min, and the temperature of the reactor was set at 60°C, and then the two reacted, and the intermediate I was collected by cooling down to room temperature through the condenser;
[0056] 2) Add 1000mL ethanol to Intermediate I and mix it evenly, pump it into the reaction column tube containing manganese dioxide (1.0 equivalent) at a flow rate of 1mL / min, inject oxygen into the reaction column at a rate of 30mL / min Oxidation reaction is carried out in the above-mentioned continuous reaction column, and the reaction temperature is set at 35° C. to obtain intermediate II;
[0057] 3) Put intermediate II and 30% hydrochloric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com