Transaminase and application thereof in preparation of (R)-alpha-methyltryptamine compound
A technology of methyltryptamine and transaminase, applied in microorganism-based methods, applications, transferase and other directions, can solve the problems of complex post-processing and high production cost, and achieve the effects of simple post-processing, high conversion rate and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] According to the enzyme gene sequence of transaminase as shown in SEQ ID NO:1, SEQ ID NO:2 and SEQ ID NO:3 (Table 1), the whole gene synthesis transaminase enzyme gene is connected into pET21a carrier at NdeI, HindIII site, It was verified by sequencing that the gene was successfully connected to the corresponding carrier. The company for gene synthesis and sequencing is Sangon Bioengineering Co., Ltd. (Shanghai) Co., Ltd. (698 Xiangmin Road, Songjiang District, Shanghai).
[0061] The vector containing the transaminase gene is transformed into the host E. coli BL21 (DE3) competent cell to obtain engineering strains respectively containing each transaminase. After the engineering bacteria containing the transaminase gene were activated by streaking on the plate, a single colony was picked and inoculated into 5 mL LB liquid medium containing 100 μg / mL ampicillin antibiotic, and cultured with shaking at 37°C for 12 hours. Transfer to 150mL fresh LB liquid medium also con...
Embodiment 2
[0064] The screening of embodiment 2 transaminases
[0065] The bacteria sludge of the three transaminases obtained in Example 1 and the triethanolamine buffer were homogenized at a mass ratio of 1:4 to obtain a transaminase enzyme liquid for use.
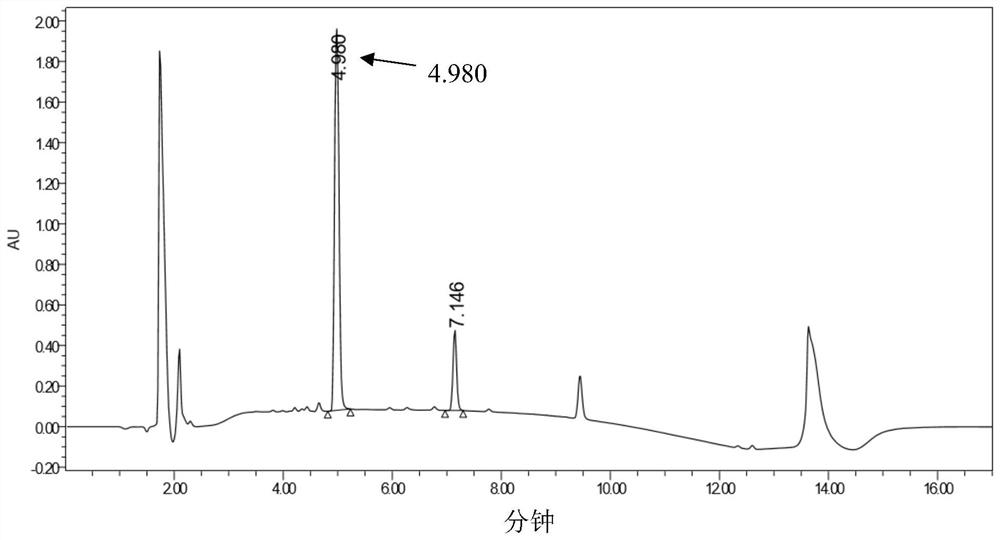
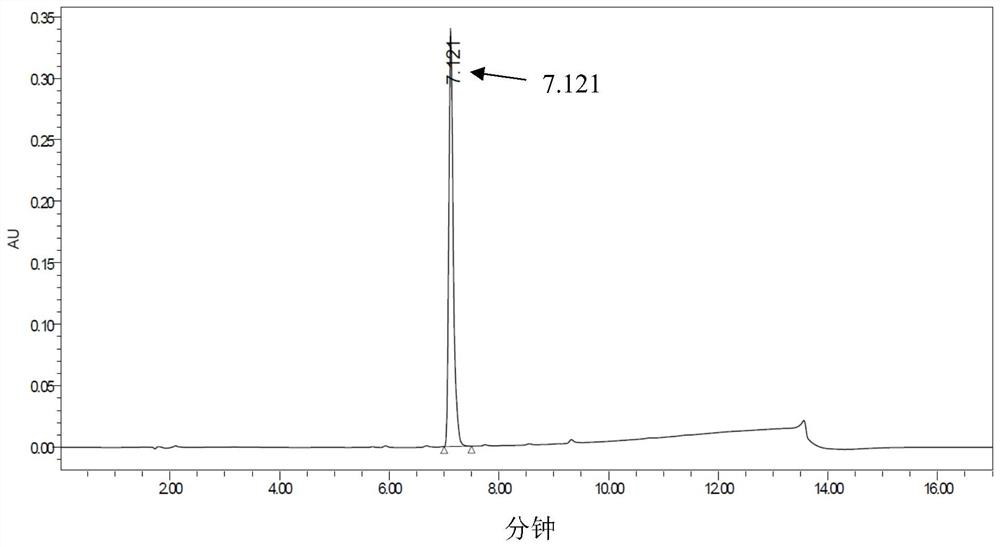
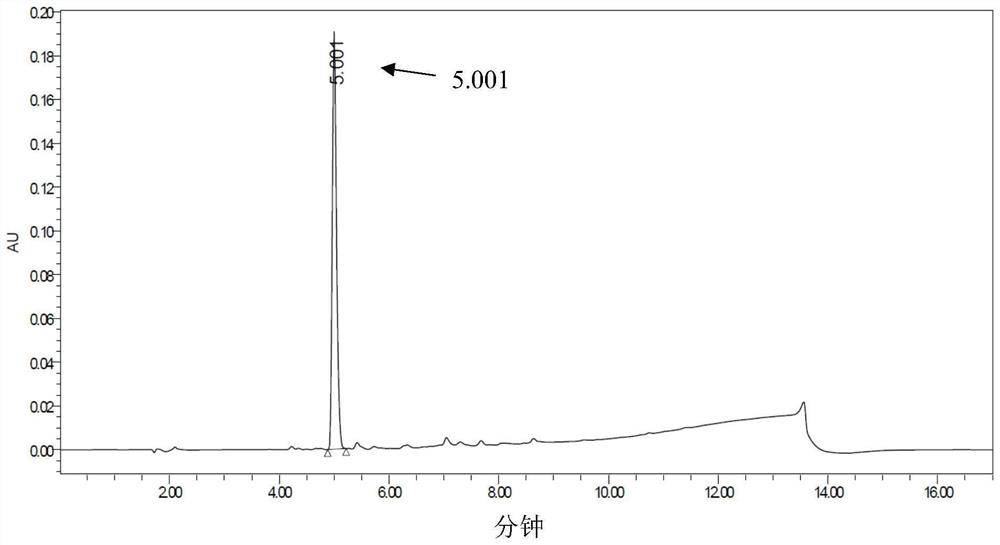
[0066] Add 625 μL of 200 mM PBS (phosphate buffered saline) with a pH of 7.0, 1 mL of transaminase enzyme solution, 100 μL of 50 mM pyridoxal phosphate (PLP), 62.5 μL of 4M isopropylamine hydrochloride, and 212.5 μL of water into the reaction vessel. Substrate (3-indolylacetone) DMSO solution (0.0086g substrate dissolved in 500μL DMSO) was added, and the reaction was carried out at 30°C. Samples were taken regularly, and the conversion rate and ee value were measured by HPLC.
[0067] The experimental results are shown in Table 2 below.
[0068] Table 2
[0069]
[0070]
[0071] \ in the table represents (because the conversion rate is too low) not detected.
[0072] It can be seen from the table that in the reaction sys...
Embodiment 3
[0073] The pH optimization in the transamination reaction of embodiment 3
[0074] Homogenize the Enz.01 bacteria sludge obtained in Example 1 and the triethanolamine buffer solution at a mass ratio of 1:4 to obtain a transaminase enzyme solution for use.
[0075] Add 0.8mL triethanolamine buffer solution with different pH values, 60mg transaminase homogenized enzyme solution, 12μL 16mg / mL pyridoxal phosphate (PLP) and 33mg isopropylamine hydrochloride in the reaction vessel, and adjust the pH to the values shown in the table. After stirring at the pH value indicated at 3, add the substrate (3-indolyl acetone) DMSO solution (20 mg substrate dissolved in 0.4 ml DMSO), react at 35°C while controlling the pH. Samples were taken regularly, and the conversion rate was measured by HPLC.
[0076] The experimental results are shown in Table 3 below.
[0077] table 3
[0078] pH 3h Conversion % 6h conversion rate% 16h Conversion % 7.5 20 31.3 40.4 8.0 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com