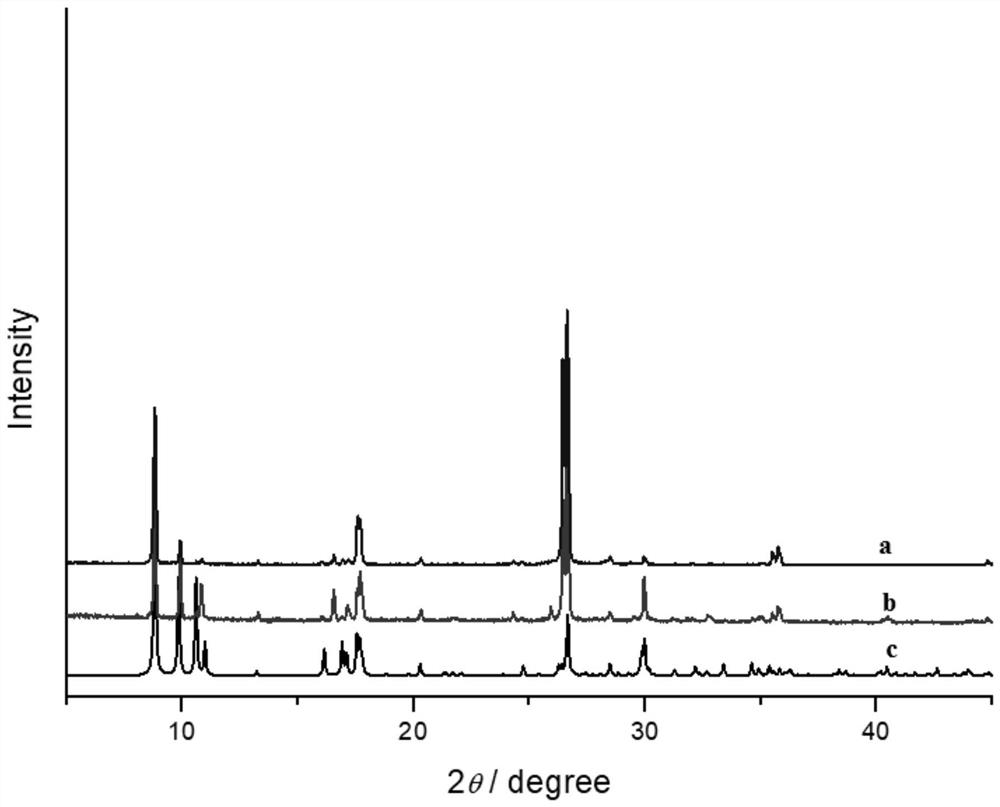
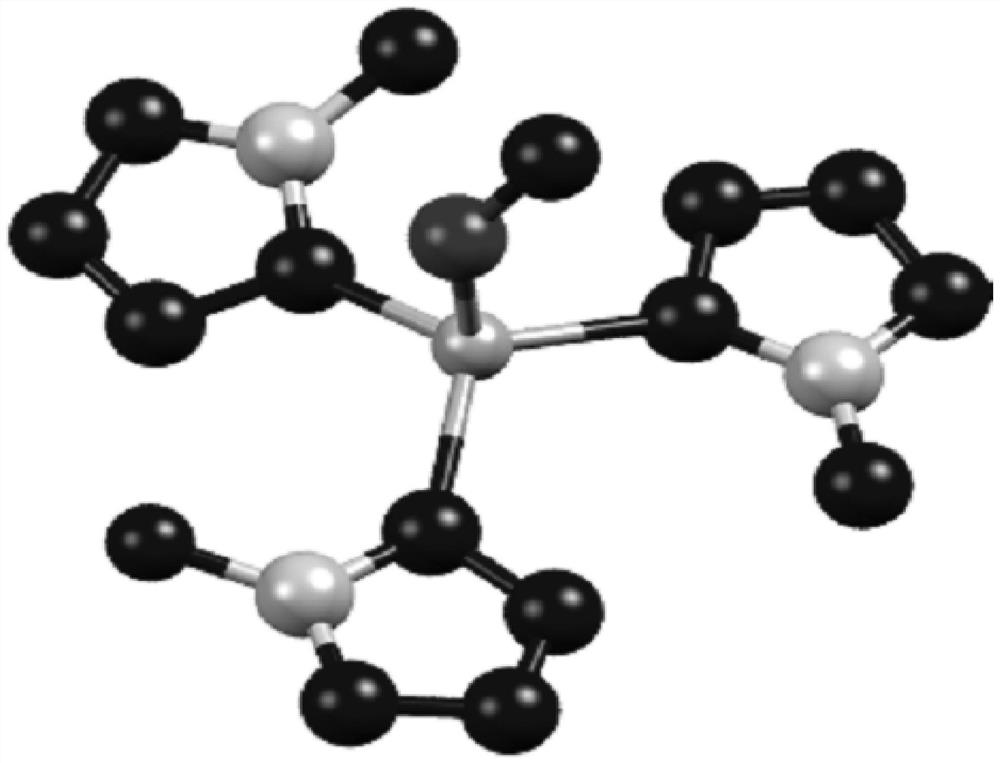
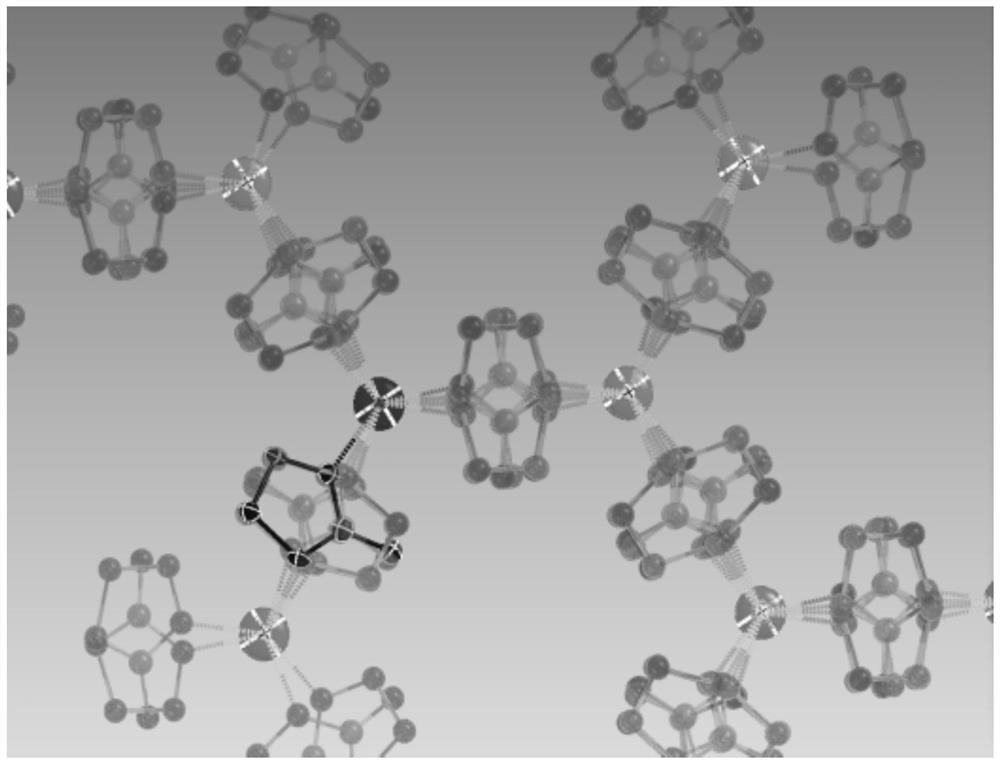
Method for for selectively adsorbing and separating nitrogen and proximity gas
A selective, gas technology, applied in separation methods, chemical instruments and methods, gas fuels, etc., can solve the problems of difficult separation of nitrogen and methane, and achieve the effects of mild synthesis conditions, simple preparation methods, and good adsorption effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present embodiment provides a kind of preparation method of zinc energetic complex of 5-aminotetrazole, and this method comprises the following steps: in 10mL there-necked flask, add 8mL N,N-dimethylformamide and 2mL water, stir Add 36.3mg (0.1mmol) of 5-aminotetrazole and 38.0mg (0.2mmol) of zinc nitrate hexahydrate, heat to 40°C to dissolve completely, and obtain a colorless clear liquid, cool to room temperature, and filter out the insoluble matter. The filtrate was placed in a clean beaker and slowly evaporated at room temperature for 7 days to obtain colorless crystals. The reaction solution was filtered off to obtain a white solid with a yield of 78%.
Embodiment 2
[0046] This embodiment provides a kind of preparation method of zinc energetic complex of 5-aminotetrazole, and this method comprises the following steps: in 250mL there-necked flask, add 80mL N,N-dimethylformamide and 20mL water, stir Add 363mg (1mmol) of 5-aminotetrazole and 380mg (2mmol) of zinc nitrate hexahydrate, heat to 40°C to dissolve completely, and obtain a colorless clear liquid, cool to room temperature, filter out the insoluble matter, and place the filtrate in a clean In a beaker, slowly volatilized at room temperature for 7 days to obtain colorless crystals. The reaction solution was filtered off to obtain a white solid with a yield of 69%.
[0047] From the comparison of Example 1 and Example 2, it can be seen that when the amount of raw materials in Example 2 is ten times larger than that of Example 1, the yield only drops from 78% of Example 1 to 69% of Example 2 , the decline is not obvious, indicating that the preparation method of the present invention h...
Embodiment 3
[0060] This example provides a method for selective adsorption and separation of nitrogen and methane gas, which uses 5-aminotetrazolium zinc energetic complex as an adsorbent to selectively adsorb and separate nitrogen and methane gas.
[0061] The 5-aminotetrazole zinc energetic complex in this example is the 5-aminotetrazole zinc energetic complex in Example 1 or 2.
[0062] The specific process is as follows:
[0063] First, solvent displacement of the target compound:
[0064] In a 100mL three-necked beaker, add 60mL of dichloromethane and 1.0g of 5-aminotetrazolium zinc energetic complex, replace the dichloromethane every 6.0h, and soak for 1d. The energetic complex of zinc 5-aminotetrazolium obtained by suction filtration was degassed at 140° C. for 12.0 h under high vacuum. The energetic complex of 5-aminotetrazole zinc after solvent replacement is obtained, and the trace moisture remaining in the target product 5-aminotetrazole zinc energetic complex is removed, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com