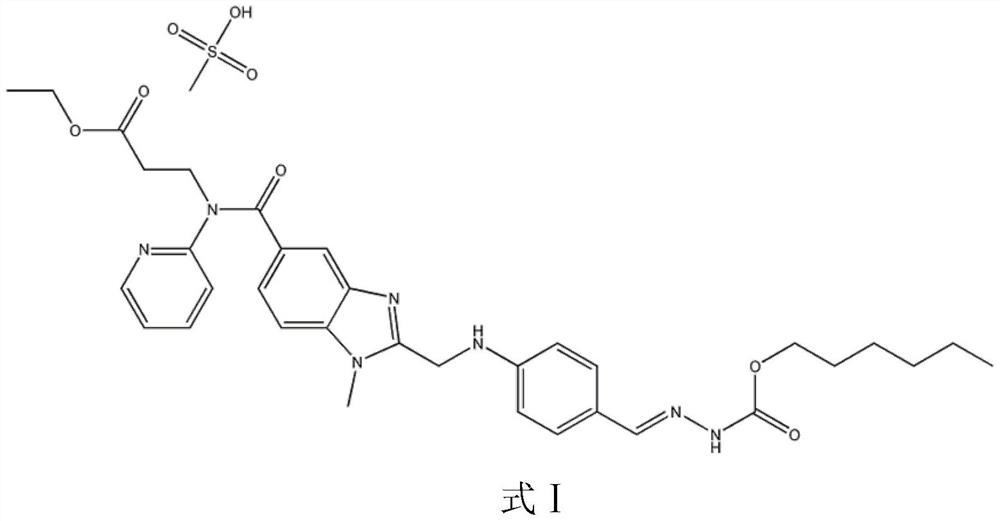
Dabigatran etexilate mesylate inclusion compound, preparation method and application
A dabigatran etexilate mesylate and inclusion compound technology, which is applied in the field of dabigatran etexilate mesylate inclusion compound, can solve the problems of low bioavailability, low drug solubility, and gastrointestinal irritation of organic acids In order to avoid the problems of large drug resistance and other problems, it can achieve the effect of reducing the dosage, having a good market prospect and improving the bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
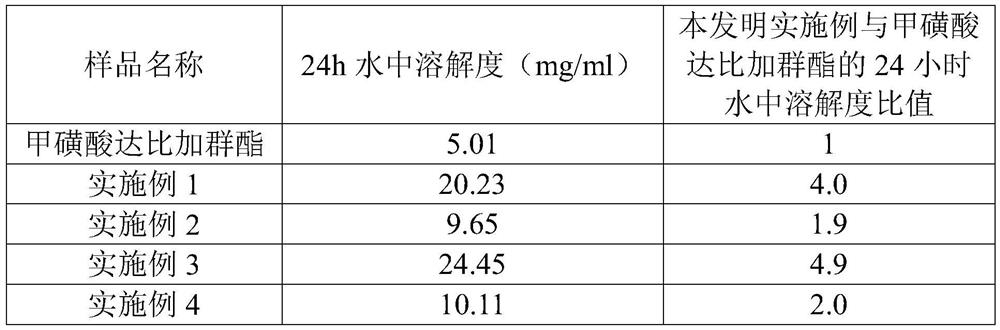
Embodiment 1
[0046]Dabigatran etexilate mesylate: hydroxypropyl-β-cyclodextrin=1:1 (molar ratio of substances)
[0047] Weigh 0.050 g of dabigatran etexilate mesylate and 0.104 g of hydroxypropyl-β-cyclodextrin respectively, and prepare clathrates according to the following method.
[0048] After dabigatran etexilate mesylate was dissolved with 1ml methanol, it was added dropwise to 9% hydroxypropyl-beta-cyclodextrin aqueous solution (the mass percentage refers to hydroxypropyl-beta-cyclodextrin aqueous solution). The mass of cyclodextrin and the percentage of the mass of hydroxypropyl-β-cyclodextrin aqueous solution), in a magnetic stirrer, 60 ° C (water bath) inclusion for 7 hours, let cool to room temperature (20 ° C ~ 25 ° C), rotary evaporation , filtered, and freeze-dried to obtain dabigatran etexilate mesylate hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 2
[0050] Dabigatran etexilate mesylate: hydroxypropyl-β-cyclodextrin=1:2 (molar ratio of substances)
[0051] Weigh 0.200 g of dabigatran etexilate mesylate and 0.832 g of hydroxypropyl-β-cyclodextrin respectively, and prepare clathrates according to the following method.
[0052] After dabigatran etexilate mesylate was dissolved with 4ml methanol, it was added dropwise to 9% hydroxypropyl-beta-cyclodextrin aqueous solution (the mass percentage refers to hydroxypropyl-beta-cyclodextrin aqueous solution). The mass of cyclodextrin and the percentage of the mass of hydroxypropyl-β-cyclodextrin aqueous solution), in a magnetic stirrer, 60 ° C (water bath) inclusion for 7 hours, let cool to room temperature (20 ° C ~ 25 ° C), rotary evaporation , filtered, and freeze-dried to obtain dabigatran etexilate mesylate hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 3
[0054] Dabigatran etexilate mesylate: sulfobutyl-β-cyclodextrin=1:1 (molar ratio of substances)
[0055] Weigh 0.200 g of dabigatran etexilate mesylate and 0.328 g of sulfobutyl-β-cyclodextrin respectively to prepare clathrates according to the following method.
[0056] After dabigatran etexilate mesylate was dissolved with 4ml of methanol, it was added dropwise to 9% sulfobutyl-β-cyclodextrin aqueous solution (the mass percentage refers to sulfobutyl-β-cyclodextrin aqueous solution). The mass of cyclodextrin and the percentage of the mass of sulfobutyl-β-cyclodextrin aqueous solution), in a magnetic stirrer, 60 ° C (water bath) inclusion for 7 hours, let cool to room temperature (20 ° C ~ 25 ° C), rotary evaporation , filtered, and freeze-dried to obtain dabigatran etexilate mesylate hydroxypropyl-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com