A kind of synthetic method of fluocinolone acetate
A technology of fluocinolone acetate and a synthesis method, which is applied in the directions of steroids, organic chemistry, etc., can solve the problems of large environmental hazards, dangerous reagents, complicated operations, etc., and achieves the effects of less environmental pollution, less risk, and optimized reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
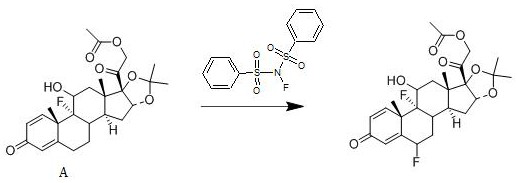
[0020] A kind of synthetic method of fluocinolone acetate, its reaction scheme is:
[0021]
[0022] The specific steps are:
[0023] Add 1 mol of compound A to dichloromethane, add 1.3 mol of N-fluorobisbenzenesulfonamide to it at 20°C, stir well, then add 0.008 mol of free radical initiator, stir for 3 hours, use 10% mass fraction The pH of the system is adjusted to be neutral with an aqueous solution of sodium hydroxide, concentrated under reduced pressure, and crystallized from ethanol to obtain fluocinolone acetate with a yield of 92.8% and a purity of 99.2%; the free radical initiator is selected from azobisisobutyronitrile.
Embodiment 2
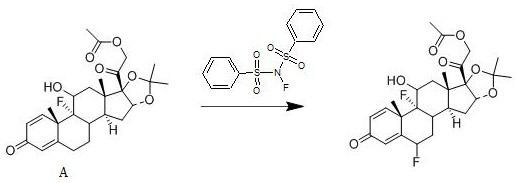
[0025] A kind of synthetic method of fluocinolone acetate, its reaction scheme is:
[0026]
[0027] The specific steps are:
[0028] Add 1 mol of compound A into dichloromethane, add 1.5 mol of N-fluorobisbenzenesulfonamide to it at 25°C, stir well, then add 0.012 mol of free radical initiator, stir for 2 hours, use 10% mass fraction Sodium hydroxide aqueous solution adjusts the pH of the system to be neutral, concentrates under reduced pressure, and ethanol crystallizes to obtain fluocinonide acetate with a yield of 95.3% and a purity of 99.5%; the free radical initiator is selected from azobisisoheptanonitrile.
Embodiment 3
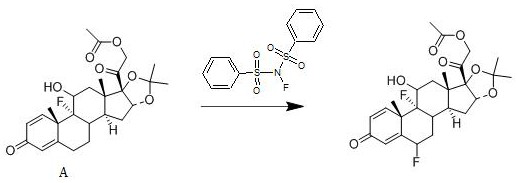
[0030] A kind of synthetic method of fluocinolone acetate, its reaction scheme is:
[0031]
[0032] The specific steps are:
[0033] Add 1 mol of compound A to dichloromethane, add 1.4 mol of N-fluorobisbenzenesulfonamide to it at 22°C, stir well, then add 0.010 mol of free radical initiator, stir for 2.5 h, and use a mass fraction of 10% The aqueous solution of sodium hydroxide was used to adjust the pH of the system to be neutral, concentrated under reduced pressure, and crystallized from ethanol to obtain fluocinolone acetate with a yield of 94.7% and a purity of 99.1%; the free radical initiator was selected from benzoyl peroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com