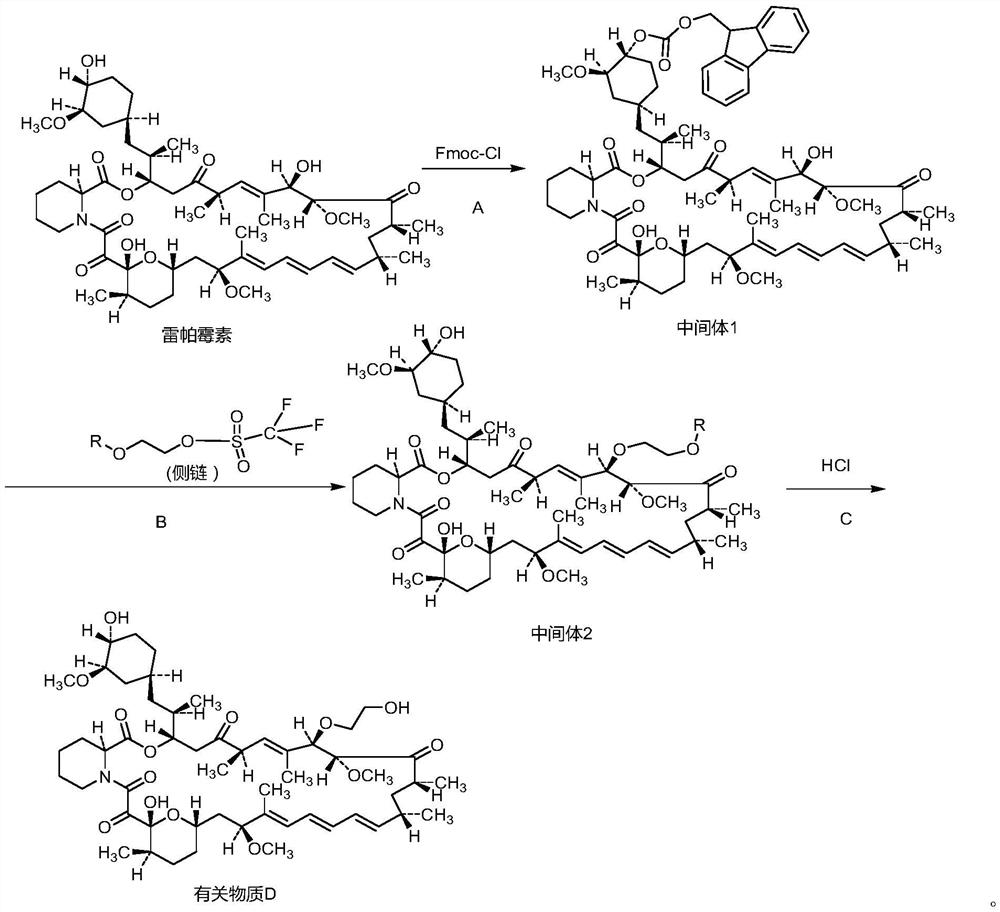
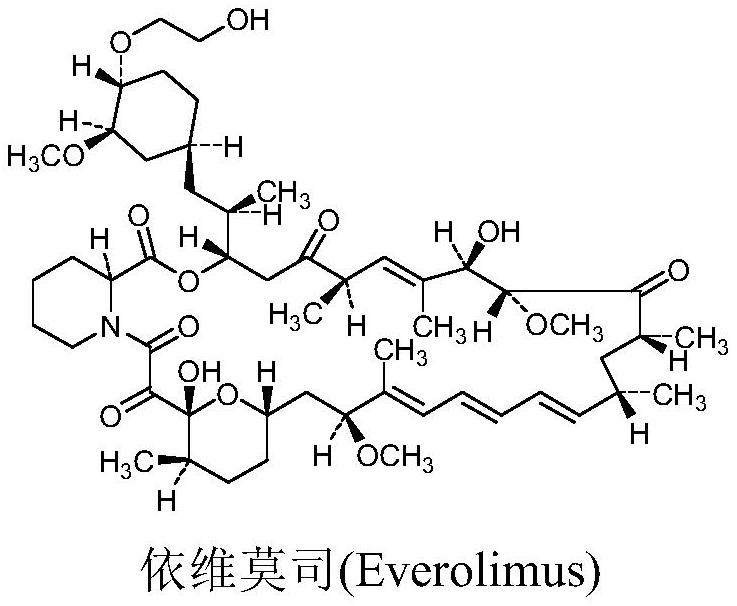
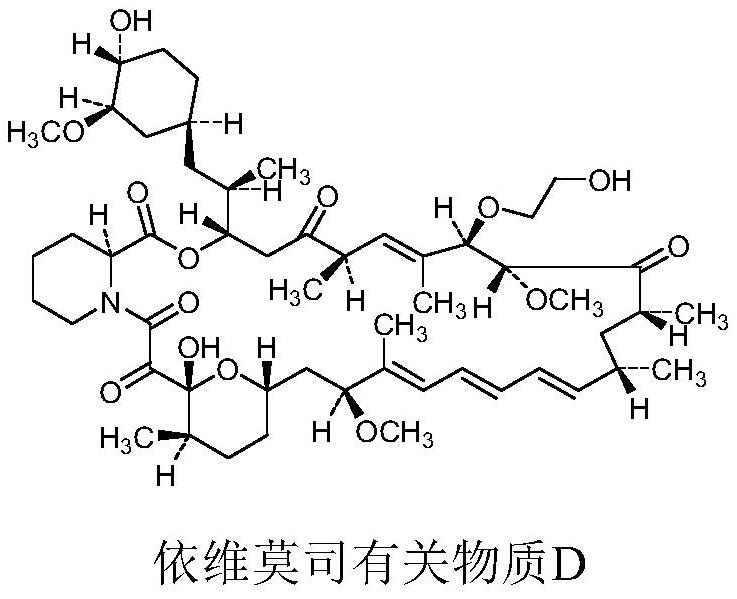
Synthesis method of everolimus related substance D
A related substance, everolimus technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of low yield, complex post-processing, etc., achieve high purity, simplify the purification process, and simplify the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis of embodiment 1 intermediate 1
[0055] Add 550ml of ethyl acetate into the round bottom flask, add 45.71g of rapamycin and 7.91g of pyridine under stirring, cool down to 0-5°C, add 15.52g of Fmoc-Cl, stir for 2 hours, raise the temperature to 25-30°C, keep warm Stir the reaction, TLC detects the reaction process until the rapamycin spots disappear, and the reaction is complete; add 150ml of purified water, stir for 20 minutes, extract and separate the liquid, extract the aqueous layer with 50ml ethyl acetate, combine the organic layers, and use 5% dilute Wash with 100ml of hydrochloric acid and 100ml of saturated brine, dry with anhydrous sodium sulfate for 4 hours, filter, and vacuum concentrate the filtrate to dryness at a temperature of 30-35°C, separate and purify on a silica gel column (respectively use petroleum ether / ethyl acetate=3 / 1 volume ratio and petroleum ether / ethyl acetate=2 / 1 volume ratio), the product eluate was concentrated in vacuo at 3...
Embodiment 2
[0056] The synthesis of embodiment 2 intermediate 1
[0057] Add 460ml of ethyl acetate to the round bottom flask, add 45.71g of rapamycin and 7.12g of pyridine under stirring, cool down to 0-5°C, add 13.58g of Fmoc-Cl, stir for 2 hours, heat up to 25-30°C, keep warm Stir the reaction, TLC detects the reaction process until the rapamycin spots disappear, and the reaction is complete; add 150ml of purified water, stir for 20 minutes, extract and separate the liquid, extract the aqueous layer with 50ml ethyl acetate, combine the organic layers, and use 5% dilute Wash with 100ml of hydrochloric acid and 100ml of saturated brine, dry with anhydrous sodium sulfate for 4 hours, filter, and vacuum concentrate the filtrate to dryness at a temperature of 30-35°C, separate and purify on a silica gel column (respectively use petroleum ether / ethyl acetate=3 / 1 volume ratio and petroleum ether / ethyl acetate=2 / 1 volume ratio), the product eluate was concentrated in vacuo at 30-35°C to drynes...
Embodiment 3
[0058] The synthesis of embodiment 3 intermediate 1
[0059] Add 640ml of ethyl acetate to the round bottom flask, add 45.71g of rapamycin and 8.70g of pyridine under stirring, cool down to 0-5°C, add 18.11g of Fmoc-Cl, stir for 2 hours, heat up to 25-30°C, keep warm Stir the reaction, TLC detects the reaction process until the rapamycin spots disappear, and the reaction is complete; add 150ml of purified water, stir for 20 minutes, extract and separate the liquid, extract the aqueous layer with 50ml ethyl acetate, combine the organic layers, and use 5% dilute Wash with 100ml of hydrochloric acid and 100ml of saturated brine, dry with anhydrous sodium sulfate for 4 hours, filter, and vacuum concentrate the filtrate to dryness at a temperature of 30-35°C, separate and purify on a silica gel column (respectively use petroleum ether / ethyl acetate=3 / 1 volume ratio and petroleum ether / ethyl acetate=2 / 1 volume ratio), the product eluate was concentrated in vacuo at 30-35°C to drynes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com