Preparation method of high-purity LCP material
A high-purity, high-temperature technology, applied in the direction of liquid crystal materials, chemical instruments and methods, etc., can solve the problems of affecting the comprehensive performance of TLCP materials, affecting the properties of TLCP, and low purity, so as to meet the needs of mass production and manufacturing, and good application Foreground, the effect of simple overall process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
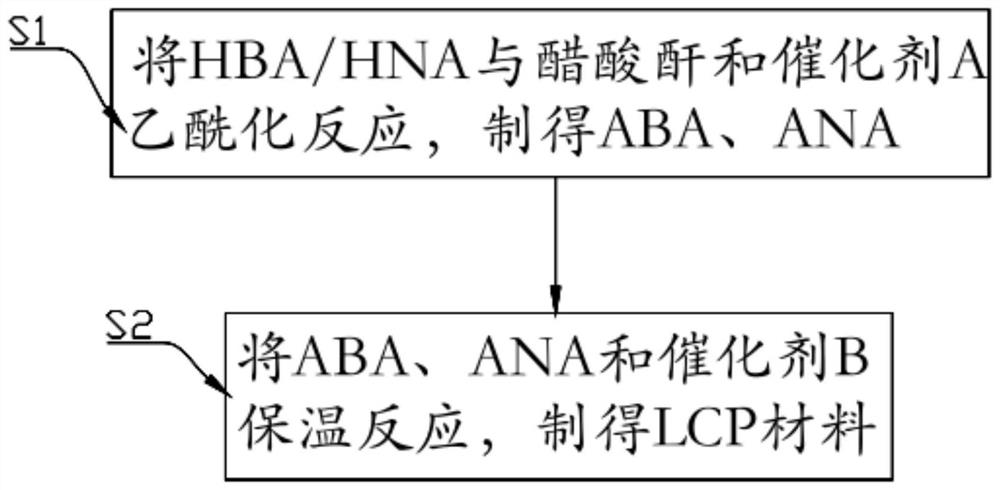
[0027] The preparation method of the high-purity LCP material of the present application comprises the following steps:
[0028] S1: Acetylation of HBA and HNA
[0029] Add HBA / HNA, acetic anhydride and catalyst A into the reaction vessel, raise the temperature to 140°C, pass through protective nitrogen gas and start stirring, the stirring speed is 200-300rpm, under this condition, HBA / HNA reacts with acetic anhydride to form ester compound, and condensed acetic anhydride to reflux for acetylation reaction, then stop the reaction, transfer the reactant to a container with deionized water, wait for the white crystals to precipitate, and finally wash and dry the white crystals for later use, the white crystals here for ABA / ANA;
[0030] S2: Preparation of high-purity LCP materials
[0031] Add the ABA, ANA and catalyst B prepared in S1 into the reaction vessel, transfer the reaction vessel to the salt bath and pass nitrogen to remove the air in the system, raise the temperatur...
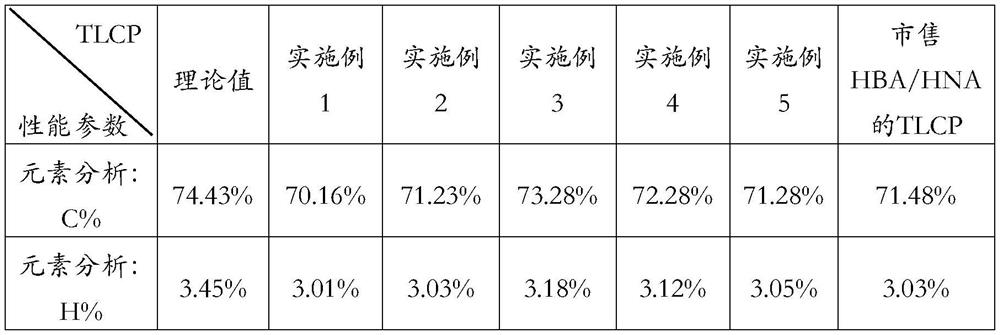
Embodiment 1
[0044] S1: Acetylation of HBA and HNA
[0045]P-hydroxybenzoic acid (HBA) and acetic anhydride with a molar ratio of 1:2 were added to the three-necked flask, and the catalyst zinc acetate of 0.05% mass fraction was added simultaneously; Turn on stirring at a stirring speed of 300rpm / min, keep warm at this temperature for 2h to carry out the acetylation reaction, transfer it to a beaker with deionized water after the reaction, wait for the crystals to precipitate, and finally wash and dry the precipitated crystals (ABA) for later use .
[0046] Add 2-hydroxy-6-naphthoic acid (HNA) and acetic anhydride with a molar ratio of 1:2 into the three-necked flask, and at the same time add 0.05% mass fraction of the catalyst 1-methylimidazole; raise the temperature to react in the three-necked flask The temperature of the system reaches 140°C and the stirring is started at the same time. The stirring speed is 300rpm / min. The acetylation reaction is carried out at this temperature for 2...
Embodiment 2
[0050] S1: Acetylation of HBA and HNA
[0051] P-hydroxybenzoic acid (HBA) and acetic anhydride with a molar ratio of 1:3 were added into the there-necked flask, and the catalyst zinc acetate of 0.08% mass fraction was added simultaneously; Turn on the stirring, the stirring speed is 300rpm / min, keep warm at this temperature for 3h to carry out the acetylation reaction, transfer it to a beaker with deionized water after the reaction, wait for the crystals to precipitate, and finally wash and dry the precipitated crystals (ABA) for later use .
[0052] Add 2-hydroxy-6-naphthoic acid (HNA) and acetic anhydride with a molar ratio of 1:3 into the three-necked flask, and at the same time add 0.08% mass fraction of the catalyst 1-methylimidazole; raise the temperature to react in the three-necked flask The temperature of the system reaches 140°C and the stirring is started at the same time. The stirring speed is 300rpm / min. The acetylation reaction is carried out at this temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com