Method for recycling 2-chloro-4-(4-chlorophenoxy) acetophenone residue
A technology of chlorophenoxy and acetophenone, applied in the field of recovery and utilization of 2-chloro-4-acetophenone residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
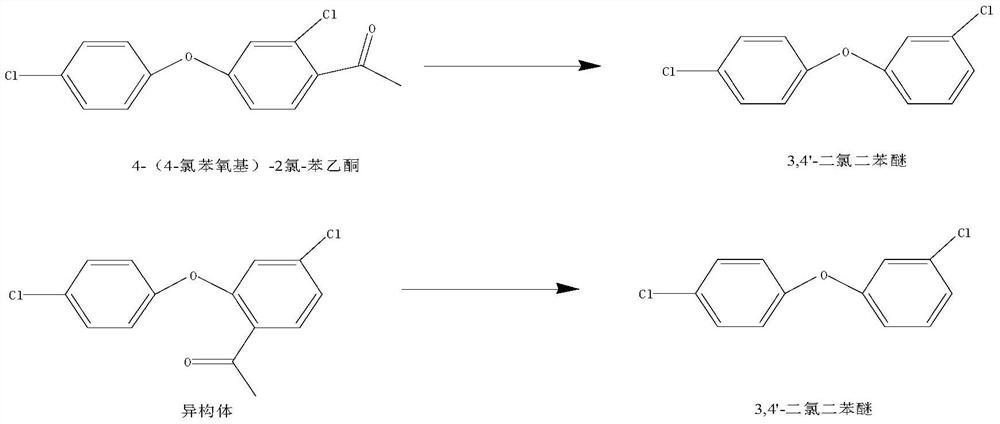
[0023] A kind of recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue
[0024] The recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue, comprises the following steps:
[0025] (1) put residue 530g, water 300g, 30wt% liquid caustic soda 250g in 2000ml autoclave, replace 3 times with nitrogen, feed nitrogen to fill pressure to 0.5MPa, then heat up to 80 ℃, pressure rise to 0.55MPa, here Under the pressure and temperature, at the time of holding the temperature for 8 hours, sampling and analysis, the content of 2-chloro-4-(4-chlorophenoxy) acetophenone and isomers is less than 3%;
[0026] (2) cooling the system temperature to 30° C., layering to obtain an organic layer and an aqueous layer;
[0027] (3) the aqueous layer is extracted with 100 g of toluene, and then the toluene layer is separated; after the toluene layer is combined with the organic layer of step (2), washed once with 50 g of water, after phase separation, toluene is recovered ...
Embodiment 2
[0031] A kind of recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue
[0032] The recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue, comprises the following steps:
[0033] (1) put residue 530g, water 300g, 30wt% liquid caustic soda 300g in a 2000ml autoclave, replace it with nitrogen 3 times, feed nitrogen to fill the pressure to 0.5MPa, then heat up to 100°C, the pressure rises to 0.6MPa, here Under pressure and temperature, keep the temperature for 6 hours, take a sample for analysis, the content of 2-chloro-4-(4-chlorophenoxy) acetophenone and isomers is less than 1%;
[0034] (2) cooling the system temperature to 30° C., layering to obtain an organic layer and an aqueous layer;
[0035] (3) the aqueous layer is extracted with 100 g of toluene, and then the toluene layer is separated; after the toluene layer is combined with the organic layer of step (2), washed with 50 g of water, after phase separation, toluene is recovered under re...
Embodiment 3
[0039] A kind of recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue
[0040] The recycling method of 2-chloro-4-(4-chlorophenoxy) acetophenone residue, comprises the following steps:
[0041] (1) put residue 580g, water 350g, 35wt% liquid caustic soda 300g in a 2000ml autoclave, replace with nitrogen 3 times, feed nitrogen to fill the pressure to 0.6MPa, then heat up to 90 ° C, the pressure rises to 0.5MPa, here Under pressure and temperature, keep warm for 7 hours, take samples for analysis;
[0042] (2) cooling the system temperature to 30° C., layering to obtain an organic layer and an aqueous layer;
[0043] (3) the water layer is extracted with 100g toluene, and then the toluene layer is separated; after the toluene layer is combined with the organic layer of step (2), washed with 65g of water, after the phase separation, the toluene is recovered under reduced pressure to no flow, and under high vacuum Evaporate to obtain 3,4'-dichlorodiphenyl ether. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com