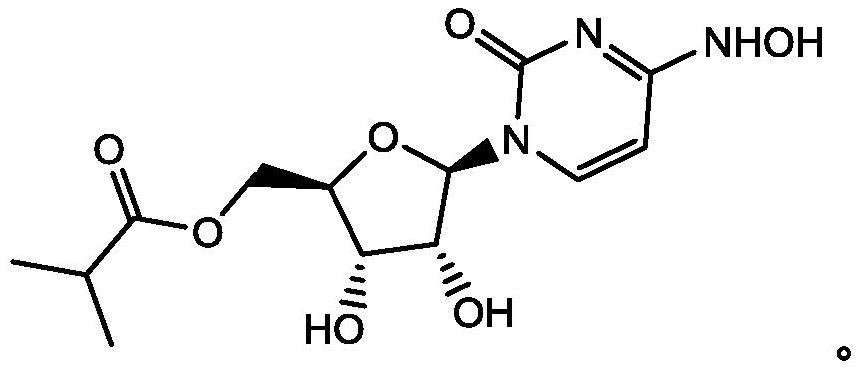
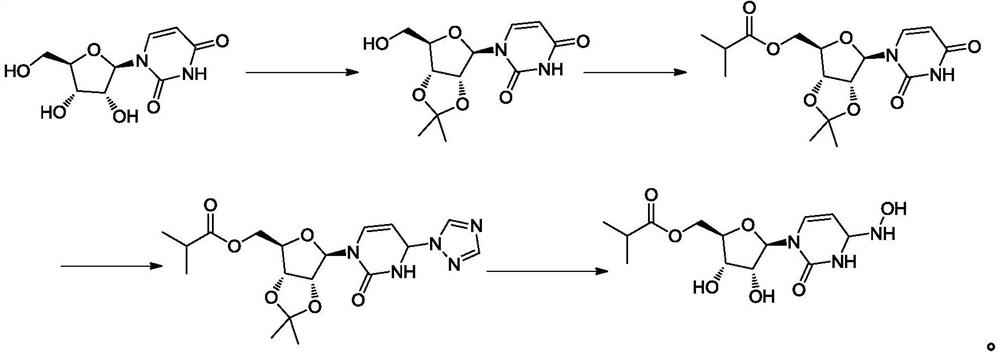
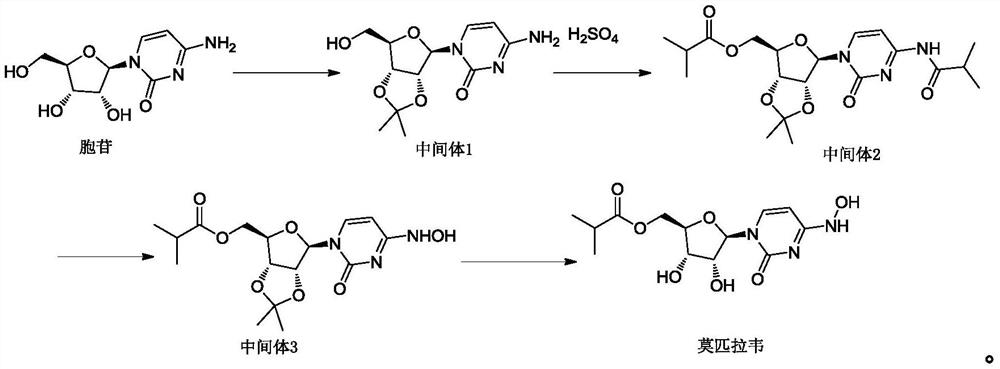
Preparation method of mupiravir
A technology of temperature control and compounding, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of lack of purity, high cost, low yield, etc., and achieve simple operation and low cost , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The present embodiment provides a kind of preparation method of mopiravir, comprising the following steps:
[0056] (1) Add the compound of formula (I) (200g, 0.47mol) in ethanol (600g), stir and dissolve, add purified water (400g), then add anhydrous sodium acetate (38.5g, 0.47mol) and hydroxylamine hydrochloride ( 65.3g, 0.94mol), heat up to 70-80°C, react for 17h, cool down to 40-45°C, add purified water (1000g), distill ethanol out under reduced pressure, cool down to 20-30°C, crystallize for 2h, filter, The filter cake was washed with a small amount of water and dried at a temperature of 50-60°C to obtain the compound of formula (II) (165g) with a purity of 99.32% and a molar yield of 94.6%;
[0057](2) The compound of formula (II) (165g, 0.45mol) was added in chloroform (825g), cooled to -5~0°C, and concentrated hydrochloric acid (67.6g, 0.67mol) was added dropwise. React for 2 hours at a temperature of 0-5°C. After the reaction is complete, add sodium carbonate ...
Embodiment 2
[0059] The present embodiment provides a kind of preparation method of mopiravir, comprising the following steps:
[0060] (1) Add the compound of formula (I) (2000g, 4.7mol) into isopropanol (6kg), stir to dissolve, add purified water (4kg), then add anhydrous sodium acetate (385g, 4.7mol) and hydroxylamine sulfate (1929g, 11.75mol), heat up to 70-80°C, react for 20h, cool down to 40-45°C, add purified water (10kg), distill isopropanol under reduced pressure, cool down to 20-30°C, crystallize for 2h, Filter, wash the filter cake with a small amount of water, and dry at a temperature of 50-60°C to obtain the compound of formula (II) (1600g), with a purity of 99.09% and a molar yield of 91.72%;
[0061] (2) The compound of formula (II) (1600g, 4.3mol) was added in dichloromethane (8kg), cooled to -5~0°C, and concentrated hydrochloric acid (438g, 4.3mol) was added dropwise. Reaction under the condition of 0~5℃ for 2h, after the reaction is completed, add ammonia water dropwise,...
Embodiment 3
[0063] The present embodiment provides a kind of preparation method of mopiravir, comprising the following steps:
[0064] (1) Add the compound of formula (I) (100kg, 235mol) in isopropanol (300kg), stir and dissolve, add purified water (200kg), then add anhydrous sodium acetate (19.3g, 235mol) and hydroxylamine sulfate ( 77.2kg, 470mol), heat up to 70-80°C, react for 20h, cool down to 40-45°C, add purified water (500kg), distill isopropanol under reduced pressure, cool down to 20-30°C, crystallize for 2h, filter , the filter cake was washed with a small amount of water, and dried at a temperature of 50-60°C to obtain a compound of formula (II) (83.5kg), with a purity greater than 99.20% and a molar yield of 95.73%;
[0065] (2) The compound of formula (II) (80kg, 216mol) is added in dichloromethane (400kg), cooled to -5~0°C, and concentrated hydrochloric acid (32.8kg, 324mol) is added dropwise. React at 0-5°C for 3 hours. After the reaction is complete, add ammonia water dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com